methemoglobinemia
Methemoglobinemia
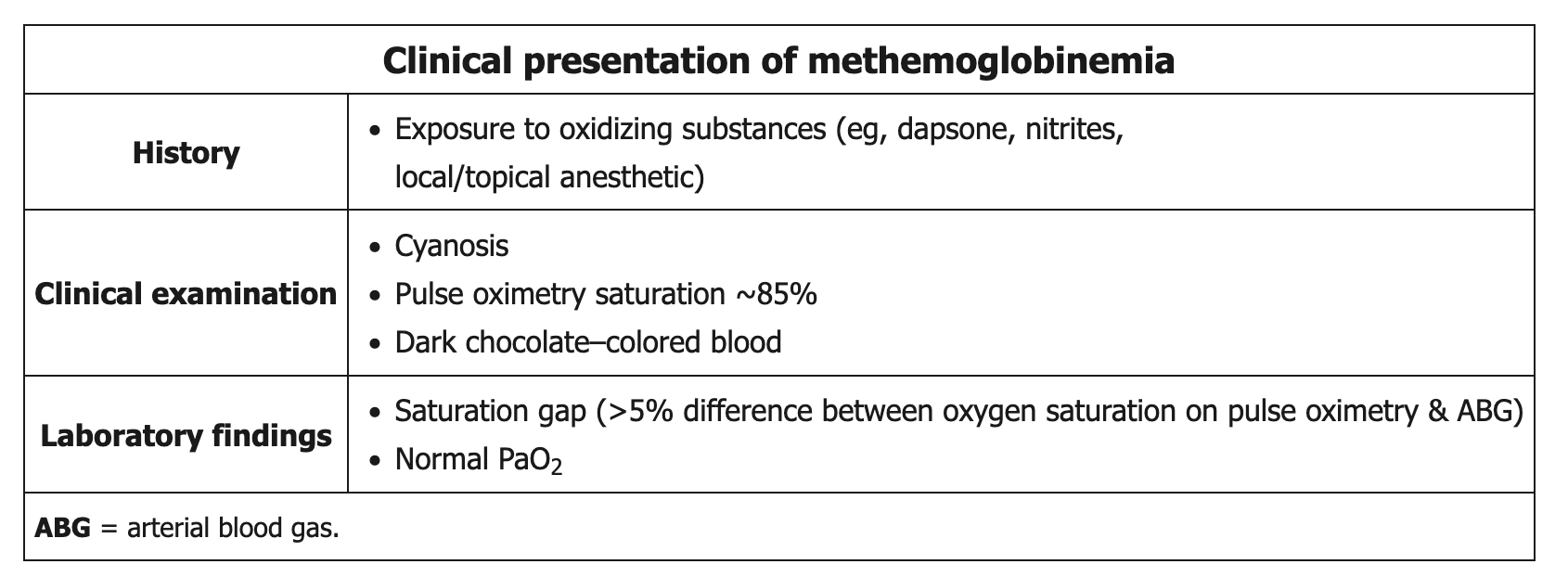 Methemoglobinemia is most commonly acquired after excessive exposure to an oxidizing agent (eg, dapsone, nitrites, local/topical anesthetics such as benzocaine/lidocaine (surgical patients)). Methemoglobin is a form of hemoglobin in which 1 of the 4 iron molecules is oxidized to the ferric (Fe3+) rather than the normal ferrous (Fe2+) state. The ferric state has a decreased affinity for oxygen, but the remaining 3 ferrous heme sites have an increased oxygen affinity, which leads to decreased oxygen delivery to peripheral tissues.
Methemoglobinemia is most commonly acquired after excessive exposure to an oxidizing agent (eg, dapsone, nitrites, local/topical anesthetics such as benzocaine/lidocaine (surgical patients)). Methemoglobin is a form of hemoglobin in which 1 of the 4 iron molecules is oxidized to the ferric (Fe3+) rather than the normal ferrous (Fe2+) state. The ferric state has a decreased affinity for oxygen, but the remaining 3 ferrous heme sites have an increased oxygen affinity, which leads to decreased oxygen delivery to peripheral tissues.
Patients typically have cyanosis and dark chocolate-colored blood. Standard pulse oximetry readings are low (85%) due to the absorption spectrum of methemoglobin and do not represent the true oxygen saturation. Due to the poor oxygen affinity of methemoglobin, the administration of supplemental oxygen does not improve cyanosis, blood color, or pulse oximetry readings. Because arterial blood gas testing analyzes unbound arterial oxygen (as opposed to hemoglobin-bound oxygen), the PaO2 is normal and overestimates the degree of true oxygen saturation. With severe cases of methemoglobinemia, the decreased oxygen delivery can result in altered mental status, seizures, and death.
Confirmation of the diagnosis requires a co-oximeter, which is a device that can accurately measure the presence of methemoglobin. The offending agent(s) should be discontinued in all patients with suspected methemoglobinemia, while symptomatic patients and those with a methemoglobin level > 20% require prompt administration of methylene blue (a reducing agent). Those in shock may also require blood transfusion or an exchange transfusion.
Treatment: Methylene blue acts as an electron acceptor for NADPH and is reduced to leucomethylene blue, which in turn reduces methemoglobin back to hemoglobin.