getational diabetes
- related: OBGYN
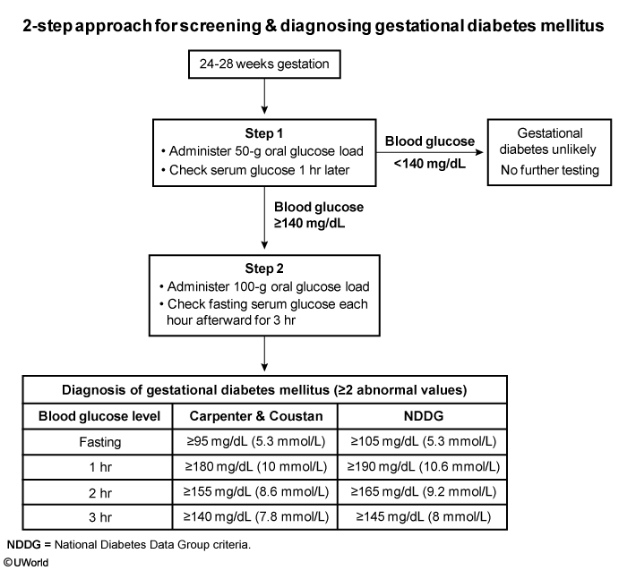
During the second and third trimesters, the placenta secretes increasing amounts of hormones (eg, human placental lactogen) that induce maternal insulin resistance. This insulin resistance ensures that there is a sufficient supply of glucose for fetal development. However, patients with inadequate pancreatic function are at risk for developing gestational diabetes mellitus (GDM). GDM is associated with obstetric (eg, preeclampsia) and neonatal (eg, hypoglycemia, polycythemia) complications. Patients with risk factors for undiagnosed pregestational type 2 DM (eg, obesity, prior macrosomic infant) are screened as early as the initial prenatal visit. Patients without risk factors are screened for GDM at 24-28 weeks gestation.
Hemoglobin A1c levels are lower in pregnant women due to physiologic increases in red blood cell mass and cell turnover. Therefore, a hemoglobin A1c is a less sensitive screening test for DM during pregnancy than a GCT.
A 2-step approach is involved in screening for GDM. The first step is a glucose challenge test (GCT), a screening test that consists of a blood glucose level measured an hour after administration of a 50-g glucose load. A blood glucose level >140 mg/dL is an indication for the second step, a 3-hour glucose tolerance test (GTT). The GTT is a diagnostic test that consists of a fasting blood glucose and blood glucose levels measured 1, 2, and 3 hours after a 100-g glucose load. GDM is diagnosed when >2 of the GTT values are elevated. This patient has an elevated GCT and requires a 3-hour GTT (Choice D).
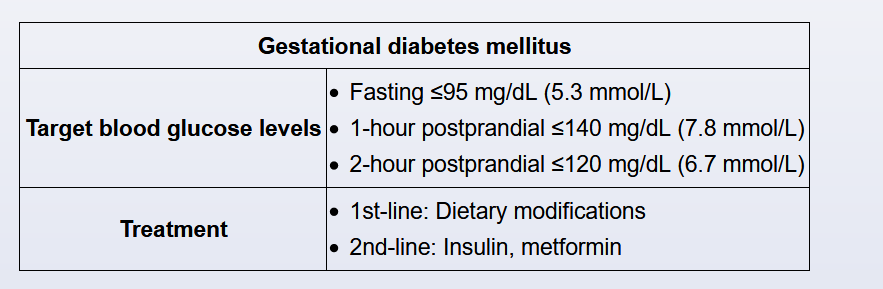
Insulin, metformin, and glyburide are all acceptable agents for control of gestational diabetes when dietary modifications do not achieve adequate glycemic control. Although there is some transplacental passage of insulin, it is not teratogenic. Metformin can cross the placenta, and the transplacental passage of glyburide is not well established; however, both medications appear to be safe in pregnancy.
Gestational diabetes mellitus requires tight glycemic control to prevent maternal and fetal complications (eg, preeclampsia, macrosomia); glycemic targets are lower than in nonpregnant patients with diabetes. The glycemic targets during pregnancy recommended by the American Diabetes Association are:
- Fasting <95 mg/dL (5.3 mmol/L)
- 1 hour postprandial <140 (7.8 mmol/L) OR 2 hours postprandial <120 mg/dL (6.7 mmol/L)
Although hemoglobin A1c measurements may be helpful, there are no clear guidelines for monitoring, and values tend to be lower in pregnant women.
Patients monitor their glucose levels at least 4 times a day (fasting and 1-2 hours postprandial). Those with moderate hyperglycemia may initially attempt dietary modification. Insulin or oral anti-hyperglycemic medications are started if patients do not achieve glycemic targets (fasting and postprandial) within a week (Choice A).
First-line management of patients with gestational diabetes mellitus is a combination of short-acting and mid- or long-acting insulin. Insulin detemir (long-acting analog) and intermediate-acting neutral protamine Hagedorn (NPH) are considered safe during pregnancy. Insulin detemir is associated with less hypoglycemia compared to NPH insulin. Human regular insulin, insulin lispro, and insulin aspart are short-acting insulins that are safe during pregnancy (insulin glargine and insulin glulisine are not approved by the US Food and Drug Administration for use during pregnancy).
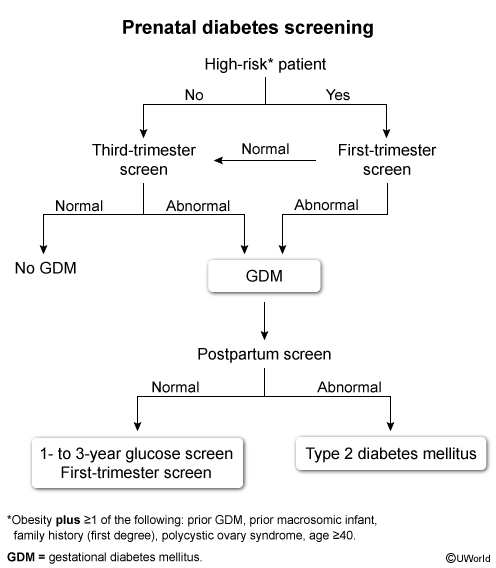
Gestational diabetes mellitus (GDM) is common in pregnancy; therefore, universal screening (ie, 1-hour glucose challenge test) is recommended at 24-28 weeks gestation. GDM is managed with dietary modifications ± insulin therapy, and resolves at delivery. However, some patients with GDM have undiagnosed type 2 diabetes mellitus; therefore, patients require screening again at 6-12 weeks postpartum with a 2-hour glucose tolerance test.
Patients with a history of GDM, even with normal postpartum testing, are at increased risk of both GDM in future pregnancies and type 2 diabetes mellitus (Choices A, C, and E). Therefore, patients with a history of GDM require frequent glucose screening (every 1-3 years) outside of pregnancy. During pregnancy, patients require first-trimester screening to evaluate for type 2 diabetes mellitus, as early diagnosis and treatment reduces adverse fetal outcomes (eg, congenital anomalies, macrosomia).