bias
- related: Biostats
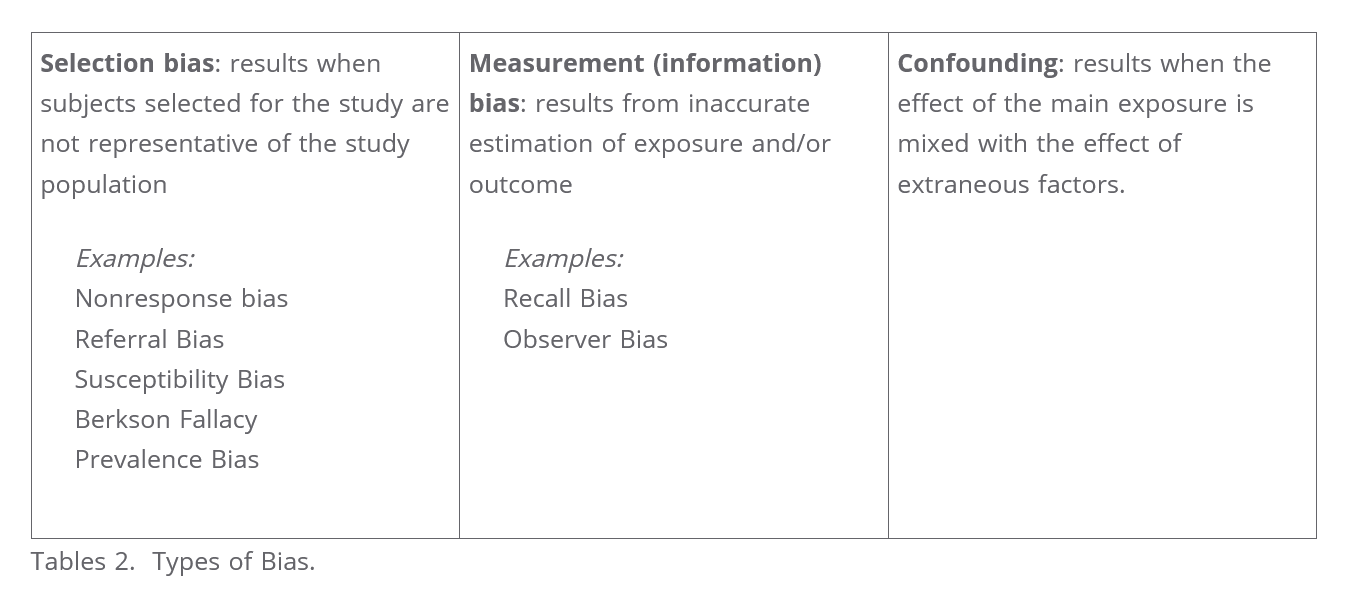
Different types of biases:
- contamination: control group receiving treatment/intervention
- observer: observer responsible for recording knows about study
- experimenter expectancy or Pygmalion effect: hopes of experimenter influence outcome
- susceptibility: prognostic difference between control/experimental
- verification: uses gold standard testing selectively to confirm positive or negative test
- sampling or ascertainment: sample does not reflect population
- attrition: loss to follow up
- measurement or hawthorne effect: people change behavior in study
- recall: difference with recall
- lead time: detect disease early
- length time: Length-time bias: Length-time bias is a phenomenon whereby a screening test preferentially detects less aggressive forms of a disease and therefore increases the apparent survival time. This is the case in Question #2, where a new screening test detects more non-aggressive prostate cancers and fewer aggressive ones than the previous method of diagnosis.
- late look: sicker subjects not responding or deceased
- prevalence-incidence or Neyman: individuals with mild or severe disease excluded
- procedure: different arms of study treated differently
Contamination bias
Contamination bias occurs when the control group unintentionally receives the treatment or the intervention, thereby reducing the difference in outcomes between the control and treatment group.
Observer bias
Observer bias occurs when an observer responsible for recording results is influenced by prior knowledge about participants or study details. Blinded studies (as in this case) usually avoid this bias by preventing observers from knowing which treatment or intervention the participants are receiving; this leads to a more objective measurement of outcomes.
Susceptibility bias
Susceptibility bias is a type of selection bias where experimental and control groups differ from a prognostic standpoint, possibly due to unforeseen confounding variables. Patients in this trial were randomly selected for a specific screening method, minimizing this type of bias.
Verification Bias
Ideally, gold standard testing would be performed in all participants; however, this may not be feasible due to ethical, time, and cost concerns, particularly if the gold standard method is invasive and associated with an increased risk of complications. For example, in a study of a new screening test for a certain liver condition where liver biopsy is the gold standard, it may not be feasible to perform a biopsy on all patients, particularly those who have a negative result on the new screening test. Such a situation can cause verification bias, or workup bias, a type of measurement bias that occurs when a study uses gold standard testing selectively in order to confirm a positive (or negative) result of preliminary testing; this can result in overestimates (or underestimates) of sensitivity (or specificity).
Ascertainment
Increased scrutiny/screening in exposed individuals
Ascertainment bias occurs when the results of a clinical study are distorted by knowledge of which intervention the participants are assigned to. Ascertainment bias can be introduced at any stage of a clinical study by individuals who: administer the interventions (eg, researchers, assistants) receive the interventions (ie, participants) ascertain the outcomes (eg, researchers, data collectors, evaluators) analyze the data (ie, statisticians) The best way to maximize unbiased ascertainment of outcomes is to keep the individuals involved in the clinical study blind (unaware) to the participants' intervention assignment for as long as possible. Although ascertainment bias is, under ideal circumstances, reduced by blinding all individuals involved in a clinical study, complete blinding cannot always be implemented (eg, in studies evaluating surgical interventions).
Ascertainment bias arises when data for a study or an analysis are collected (or surveyed, screened, or recorded) such that some members of the target population are less likely to be included in the final results than others.
Prevalence Incidence
If, when studying cardiac arrest, one only collects data on arrival the the emergency department, you would miss all the patients who were declared dead on scene, who may be systematically different from those who make it to hospital.
If you were studying risk factors for myocardial infarction among patients admitted to a cardiac ward, you could get a skewed result if you failed to include healthier patients (perhaps those who has “silent MIs”) or sicker patients, such as those who have already died from a cardiac arrest.
Length-time bias
Length-time bias is a phenomenon whereby a screening test preferentially detects less aggressive forms of a disease and therefore increases the apparent survival time.