Kidney Manifestations of Deposition Diseases
- related: Nephrology
- tags: #nephrology
Overview
Various kidney diseases are associated with deposition of immunoglobulin (Ig) and non-Ig proteins. On electron microscopy, these deposits can be unstructured or organized into fibrils or tubules (Figure 14). Monoclonal Ig deposits may be caused by myeloma, Waldenström macroglobulinemia, or chronic lymphocytic leukemia, or by the clonal expansion of Ig-secreting cells that do not meet the strict definition of these disorders, which has been termed monoclonal gammopathy of renal significance (MGRS). The pathological findings associated with monoclonal Ig deposition can include proliferative glomerulonephritis, AL amyloid, type 1 cryoglobulinemia, and, occasionally, immunotactoid and fibrillary glomerulopathy. Polyclonal Ig deposits include mixed cryoglobulinemia, whereas non-Ig proteins contribute to AA amyloid and to most cases of immunotactoid and fibrillary glomerulopathy.
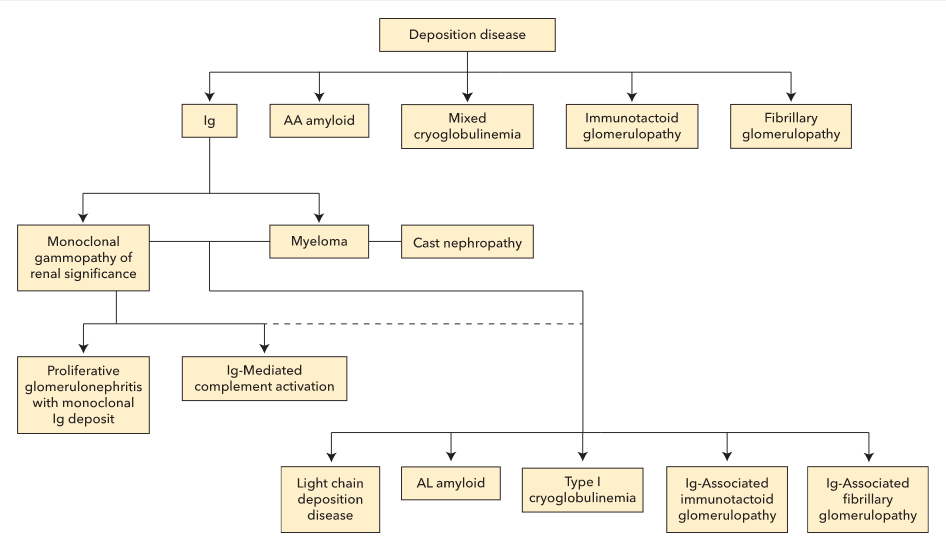 Deposition diseases can be divided into five major categories: immunoglobulin (Ig)-related disease, amyloid, cryoglobulinemia, immunotactoid glomerulopathy, and fibrillary glomerulopathy. Ig-related disease can be further divided into multiple myeloma and monoclonal gammopathy of renal significance (MGRS). Although some histopathological findings occur more commonly in either multiple myeloma (such as cast nephropathy) or MGRS, most have been reported in either disorder.
Deposition diseases can be divided into five major categories: immunoglobulin (Ig)-related disease, amyloid, cryoglobulinemia, immunotactoid glomerulopathy, and fibrillary glomerulopathy. Ig-related disease can be further divided into multiple myeloma and monoclonal gammopathy of renal significance (MGRS). Although some histopathological findings occur more commonly in either multiple myeloma (such as cast nephropathy) or MGRS, most have been reported in either disorder.
Monoclonal Immunoglobulin–Associated Kidney Disease
A wide array of renal lesions can be caused by clonal expansion of Ig-secreting cells. If there are greater than 10% plasma cells on bone marrow biopsy or the presence of an extramedullary plasmacytoma, a diagnosis of multiple myeloma can be made. However, as noted above, paraproteinemia caused by clonal expansion of Ig-secreting cells that do not meet the strict definition of myeloma, Waldenström macroglobulinemia, or chronic lymphocytic leukemia can be associated with nephrotoxicity. Although some of the findings noted pathologically occur more frequently with either MGRS or myeloma, except for myeloma cast nephropathy seen exclusively with myeloma, all the renal lesions can occur in each of these disorders. Because filtered light chains are endocytosed by proximal tubule cells and are nephrotoxic, patients may present with proximal tubular dysfunction with Fanconi syndrome; common features include glycosuria, phosphaturia, and normal anion gap metabolic acidosis. Kidney function may be normal or abnormal.
Myeloma Cast Nephropathy
Myeloma cast nephropathy occurs in the presence of a markedly increased concentration of free light chains and is always associated with multiple myeloma. Light chains are freely filtered by the glomerulus and combine with secreted Tamm-Horsfall protein to form obstructing casts. These tubular casts have a characteristic fractured appearance on microscopic examination. Patients with cast nephropathy present with acute or slowly progressive kidney injury. Serum free light chains are extremely elevated. Treatment is aimed at reducing the concentration of free light chains using chemotherapy. Plasmapheresis may also be beneficial in selected patients.
Monoclonal Gammopathy of Renal Significance
Monoclonal gammopathy of renal significance (MGRS) is characterized by kidney damage caused by monoclonal Ig that is secreted by a B cell or plasma cell clone not meeting diagnostic criteria for multiple myeloma or a lymphoproliferative disorder. All pathological findings associated with the clonal expansion of Ig-secreting cells can be seen in both myeloma and MGRS. The underlying pathology is likely determined by the characteristics of the secreted protein. Entities associated with MGRS include proliferative glomerulonephritis with monoclonal Ig deposits (PGNMID), C3 glomerulopathy with monoclonal gammopathy, AL amyloid, type 1 cryoglobulinemia, fibrillary glomerulonephritis, and immunotactoid glomerulopathy.
Kidney manifestations of MGRS are usually caused by deposition of monoclonal Ig light chains. Patients can present with both nephrotic and subnephrotic proteinuria, hematuria, and elevated serum creatinine.
Kidney biopsy is necessary to make the diagnosis. In addition to evaluating for underlying myeloma and chronic lymphocytic leukemia, further testing may include serum and urine protein electrophoresis, immunofixation, measurement of free light chains, and bone marrow biopsy. Because treatment is aimed at eradication of the expanded clonal line, it is important that the care of patients with MGRS be coordinated with a myeloma specialist.
Amyloidosis
Amyloidosis is a disorder characterized by the fibrillary deposition of insoluble amyloid proteins that form β-pleated sheets, which exhibit green birefringence on polarizing microscopy when stained with Congo red. Amyloid fibrils are approximately 10 nm in diameter, as opposed to the larger microtubules seen in fibrillary glomerulonephritis and immunotactoid glomerulopathy. Although amyloid can be made up of numerous proteins, kidney disease is most commonly caused by AL amyloid or AA amyloid. AL amyloid is composed of monoclonal λ (most commonly) or κ light chains produced by either MGRS or myeloma; AA amyloid is formed by serum amyloid A protein, an acute phase reactant produced in various inflammatory diseases such as rheumatoid arthritis, inflammatory bowel disease, chronic osteomyelitis, and familial Mediterranean fever. Patients with renal amyloid frequently present with nephrotic-range proteinuria. Less commonly, amyloid can affect only the renal vasculature or tubular-interstitium and have minimal or no proteinuria. Treatment is aimed at the underlying disease.
Monoclonal Immunoglobulin Deposition Disease
Monoclonal light chains (usually κ) or heavy chains can be deposited in the kidney and manifest as proteinuria and kidney failure. Unlike AL amyloid, these proteins do not form β-pleated sheets and do not stain with Congo red. These deposits can be limited to the basement membrane, giving a microscopic appearance similar to that of diabetic nodular sclerosis (light chain deposition disease), or can activate complement and induce a proliferative glomerulonephritis (PGNMID). In some cases, an underlying plasma cell dyscrasia or lymphoproliferative disorder can be identified, but criteria for myeloma or chronic lymphocytic leukemia are often absent.
Cryoglobulinemia
Of the three types of cryoglobulinemia, kidney involvement occurs most frequently with type II (mixed Ig) cryoglobulinemia and is usually associated with hepatitis C virus infection. Patients can present with a nephritic picture: elevated serum creatinine, hypertension, proteinuria, and hematuria. Membranoproliferative glomerulonephritis is usually noted on biopsy. Occasionally, a rapidly progressive glomerulonephritis with crescent formation can occur. A palpable purpuric rash is frequently present on the lower extremities. Because hepatitis C is the predominant cause, treatment is aimed at eradication of the virus.
Fibrillary and Immunotactoid Glomerulopathy
Although rare, both fibrillary and immunotactoid glomerulopathy are becoming increasingly recognized causes of kidney disease. These diseases are caused by glomerular deposition of microtubular structures that are larger than amyloid (20 nm in fibrillary glomerulopathy and >30 nm in immunotactoid glomerulopathy) and do not stain with Congo red. Proteinuria, frequently in the nephrotic range, and hematuria are common. Although the pathogenesis of these entities is unknown, both have been associated with paraproteinemia. Treatment is usually unsuccessful, and kidney outcomes are poor.