IgA nephropathy
- related: Nephrology
- tags: #nephrology
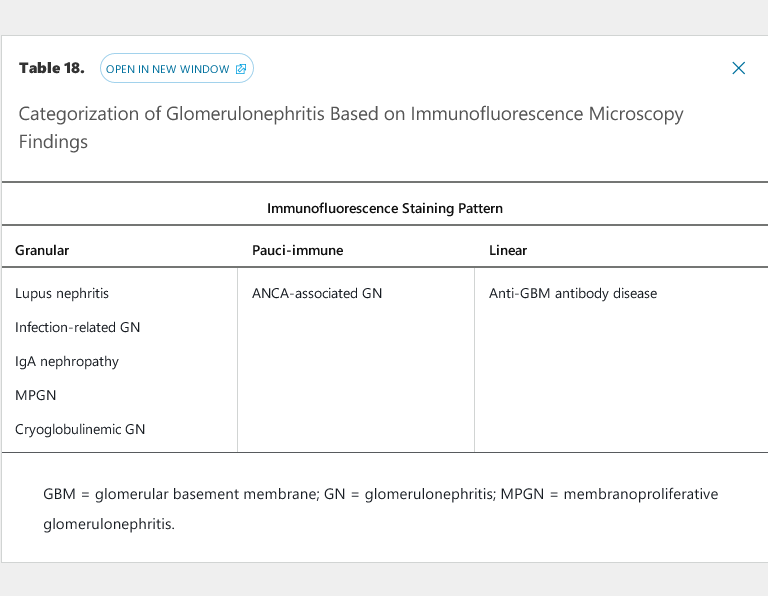
The glomerulonephritides with granular immunofluorescence microscopy staining patterns all fall under the umbrella category of immune complex–mediated glomerulonephritis, with antigen-antibody complexes depositing in the kidney at various locations and in various patterns (see Table 18). Immune complex deposition activates the classic complement pathway as a co-player in glomerular inflammation, injury, and, if unchecked, scarring. For this reason, most immune complex–mediated glomerulonephritides are associated with low levels of C3 and/or C4 (see Table 19). IgA nephropathy, which usually presents with normal C3 and C4 levels, is an exception due to the chronic, gradual nature of the disease; complement consumption is at a rate slow enough for hepatic production to replace complement proteins. In rare hyper-acute cases of IgA nephropathy that fulfill criteria for RPGN, however, complement levels are usually depressed.
IgA nephropathy (IgAN) is the most common primary glomerular disease worldwide, diagnosed in up to 10% of all kidney biopsies done in the United States and approximately one third of all kidney biopsies in Asian countries, where the disease is the leading cause of ESKD. In contrast, IgAN is very rare among patients of African ancestry. IgAN can be diagnosed at any age, particularly because a significant subgroup of patients is asymptomatic, but is most commonly diagnosed in youth or early adulthood, with a 2:1 male-to-female ratio. In the United States, the overall incidence of IgAN is estimated at 2.5 cases per 100,000 person-years, with a prevalence of approximately 80,000 individuals.
In IgAN, there is defect in IgA1-producing cells leading to hypogalactosylation of the hinge region of IgA1. Blood levels of galactose-deficient IgA1 (Gd-IgA1) are elevated in patients with IgAN. Patients then develop antibodies directed against Gd-IgA1 to form immune complexes that accumulate in the glomerular mesangium.
Symptom
IgAN can present with any of the manifestations of the nephritic syndrome. The mildest presentation, seen in up to one third of patients, is asymptomatic microscopic hematuria with or without proteinuria, usually discovered incidentally as part of a routine examination. Recurrent gross hematuria, in which macroscopic hematuria occurs in the setting of an upper respiratory infection (synpharyngitic hematuria) is a common presentation in younger patients; it usually portends a benign clinical course with recurrent episodes of gross hematuria without progression to CKD. IgAN can also present as an acute glomerulonephritis or an even more aggressive RPGN, with variable degrees of AKI, hypertension, edema, proteinuria, and hematuria.
Diagnosis
The diagnosis of IgAN may be suspected clinically but can only be made by kidney biopsy showing dominant mesangial immune-deposits of IgA with C3, and occasionally IgG or IgM. In some centers, patients with microscopic hematuria, normal kidney function, and proteinuria <500 mg/24 h are given an empiric diagnosis of IgAN without a biopsy because the disease course is expected to be mild in these cases. In other centers, patients with microscopic hematuria that is deemed to be glomerular in origin (that is, presence of dysmorphic erythrocytes or erythrocyte casts in the urine sediment) elicits a biopsy regardless of kidney function or proteinuria.
Treatment and Prognosis
There are no current standard care therapies for IgAN other than conservative, nonimmunomodulatory therapies. Antiproteinuric therapy using an ACE inhibitor or ARB is a hallmark for treating IgAN; it is usually coupled with lipid-lowering therapy and fish oil, although the efficacy of the latter two as specific IgAN therapies is unproven. The use of immunosuppression in IgAN remains controversial. In the United States, it is common for most patients with IgAN who progress to ESKD to have done so despite long-term renin-angiotensin system blockade and at least one course of immunosuppressive therapy.
Approximately one third of patients with IgAN will have a benign long-term course, with continued microscopic hematuria but little to no proteinuria and no evidence of kidney dysfunction. The disease progresses to CKD or ESKD in the remainder of patients, with up to 40% reaching ESKD within 15 years of diagnosis. The recent Oxford histopathology classification of IgAN provides evidence that advanced disease chronicity, manifesting as >50% tubular atrophy and interstitial fibrosis on kidney biopsy, is the most reliable predictor of progression to ESKD. Among clinical parameters, proteinuria ≥1000 mg/24 h has been shown to be a risk factor for progression.
Studies on using immunosuppression (particularly glucocorticoids) have used this 1000 mg/24 h proteinuria threshold for enrollment and have demonstrated conflicting results. Although earlier small studies have shown a clear benefit in adding glucocorticoids (oral or a combination of intravenous and oral) to ACE inhibitors or ARBs when proteinuria is >1000 mg/24 h, more recent larger studies (STOP-IgAN and TESTING studies) have raised concerns about the toxicity of this treatment strategy outweighing any potential benefits. Therefore, the use of immunosuppression in IgA nephropathy remains controversial.
IgA Vasculitis
IgA vasculitis (Henoch-Schönlein purpura) can present with the classic tetrad of rash, arthralgia, abdominal pain, and kidney disease. Considered a systemic version of IgAN, this vasculitis is often diagnosed empirically in the pediatric setting, because the differential diagnosis of vasculitis in children is extremely limited. In adults, the diagnosis should be confirmed with tissue biopsy (kidney or skin), because the presentation of IgA vasculitis can also fit with a non-IgA form of vasculitis. Skin biopsy is usually adequate to make the diagnosis when lesions less than 24 hours old in appearance are sampled. Kidney involvement in IgA vasculitis typically is more severe in adults than in children, with higher rates of AKI and the nephrotic syndrome. Treatment includes glucocorticoids and, in cases of associated RPGN, cyclophosphamide.