HIV testing
- related: HIV
The CDC, American College of Physicians, Infectious Diseases Society of America, and U.S. Preventive Services Task Force (USPSTF) recommend universal screening for HIV in all adults at least once. The USPSTF suggests those at higher risk (injection drug users and their sexual partners, people who exchange sex for money or drugs, sexual partners of HIV-infected persons, and those with more than one sexual partner since their most recent HIV test) should undergo repeat HIV testing at least annually. In 2017, the CDC reaffirmed its support for this recommendation but noted that clinicians can consider the potential benefits of more frequent HIV screening (for example, every 3 or 6 months) for some asymptomatic sexually active men who have sex with men based on their individual risk factors, local HIV epidemiology, and local policies.
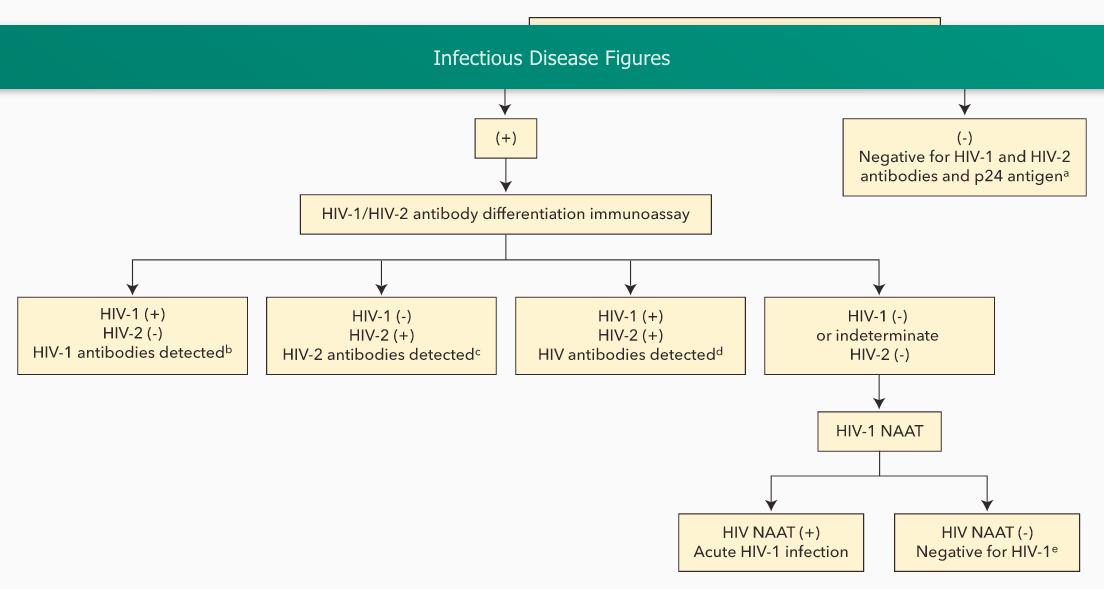
Current (fourth generation) HIV testing uses a combination assay for HIV antibody and HIV p24 antigen, which detects acute infection at least 1 week earlier than older assays. A positive result on the combination assay leads to testing with an HIV-1/HIV-2 antibody differentiation immunoassay, which, if positive, confirms infection. Specimens that test positive on the initial combination assay but negative for HIV antibody are tested for HIV RNA by nucleic acid amplification testing; if positive, acute HIV infection is confirmed. Although the initial combination assay has a 99.6% specificity, testing in low prevalence populations (such as general screening) can still result in false positives, so waiting for the results of the confirmatory antibody differentiation immunoassay and nucleic acid amplification testing is important for a definitive diagnosis.