HIV and pregnancy
- related: HIV
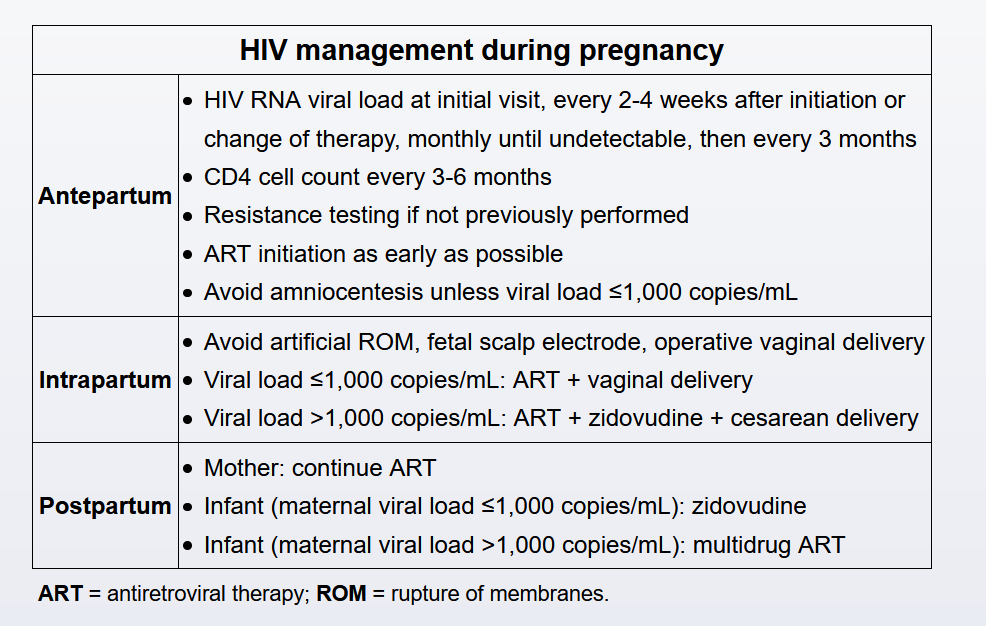
The risk of perinatal HIV transmission directly correlates with maternal HIV viral load. Antiretroviral therapy (typically, highly active antiretroviral therapy [HAART]) is continued throughout pregnancy as it is safe, treats maternal disease, and prevents perinatal transmission by suppressing viral replication and decreasing the maternal viral load.
Modification of an effective antiretroviral regimen is generally avoided due to the risk of emerging drug resistance, loss of viral suppression, and during pregnancy, perinatal transmission (Choices C and D). However, changes are indicated if significant side effects or inadequate viral suppression is present. During pregnancy, alterations in drug metabolism and distribution volume often necessitate changes in dosing or medication to maintain a low viral load. Therefore, viral loads and CD4 counts are evaluated every 3 months.
Although most transmission in untreated women occurs at the time of delivery, in utero transmission also occurs, and maintaining therapy throughout pregnancy or starting therapy immediately is important to significantly reduce the risk. Although concerns have been raised regarding the safety of efavirenz and tenofovir disoproxil fumarate in pregnancy, more recent data demonstrate the safety of these agents, including in the first trimester. A woman whose HIV is well controlled and is found to be pregnant should continue the same regimen unless another reason exists to change it.
Zidovudine, lamivudine, and ritonavir-boosted lopinavir is a valid alternative for treating HIV; it was previously a preferred regimen in pregnancy. However, changing this patient's therapy, which is well tolerated and controls her HIV infection well, is unnecessary and would only risk new adverse effects, poor adherence (because of more pills or more frequent dosing), or treatment failure.
Current guidelines recommend that all pregnant women with HIV begin taking combination antiretroviral therapy (ART) as soon as possible, regardless of initial viral load or CD4 counts, to minimize maternal risks of HIV infection and reduce perinatal transmission (Choices B and C). Drug-resistance testing is performed prior to treatment initiation; however, ART is begun immediately and modified according to results.
Combination drug therapy is more effective than single drug therapy and should include a nucleoside reverse transcriptase inhibitor and either a protease or an integrase inhibitor. Early initiation of multidrug ART allows for optimal viral load reduction by the time of delivery as viral load dictates the mode of delivery:
- Patients with viral loads ≤1,000 copies/mL may deliver vaginally without intrapartum zidovudine (AZT).
- Patients with viral loads >1,000 copies/mL receive AZT intrapartum + cesarean delivery.
Infants receive postexposure prophylaxis after delivery to further reduce their risk of viral transmission. Therapy and dosing varies based on the maternal viral load and specific infant risk factors. Mothers with HIV who live in areas where formula is readily available (eg, United States) should not breastfeed as HIV can be transmitted through breast milk. However, mothers with HIV living in developing countries should continue ART and breastfeed for 6 months to minimize infant morbidity and mortality from infectious diseases.