Disorders of the Thyroid Gland
- related: Endocrine
- tags: #endocrine
Thyroid Anatomy and Physiology
The thyroid gland is the largest dedicated endocrine organ. The usual anatomic location of the isthmus is just anterior to the second to fourth tracheal rings. The parathyroid glands are often located posterior to the superior and middle portions of each thyroid lobe. The recurrent laryngeal nerves, which innervate most of the intrinsic muscles of the larynx, course behind the thyroid gland. Thyroid pathology may cause compressive symptoms including shortness of breath, cough, dysphagia, and voice changes due to the close proximity of the thyroid to the trachea, esophagus, and recurrent laryngeal nerves.
The thyroid gland contains thyroid follicular cells and parafollicular cells (c-cells). Calcitonin is produced by the parafollicular cells and inhibits bone resorption; however, it plays a minor role in bone physiology. Follicular cells produce the thyroid hormones, thyroxine (T4) and triiodothyronine (T3). The synthesis and secretion of thyroid hormones is tightly regulated by the hypothalamic-pituitary-thyroid axis. Thyrotropin-releasing hormone (TRH) from the hypothalamus triggers the pulsatile release of thyroid-stimulating hormone (TSH) from thyrotrope cells in the anterior pituitary gland. TSH, through activation of the TSH-receptor, stimulates thyroid cell growth, iodide metabolism, and thyroid hormone synthesis and secretion. T4 and T3 exert negative feedback on the hypothalamus and pituitary gland to moderate further hormone synthesis.
Iodine is an essential dietary micronutrient and a key structural component of T4 and T3.
The thyroid gland is the exclusive source of T4, whereas approximately 80% of T3 is the result of removing one iodine molecule from T4, through deiodinase activity in peripheral tissues. This occurs primarily in the liver and kidney. Most of T4 (99.96%) and T3 (99.6%) are bound to proteins in serum. Approximately 70% of T4 and T3 are bound to thyroxine-binding globulin. Albumin, transthyretin, and lipoproteins carry a smaller proportion. Only the tiny amount of free T4 and T3 is biologically active. While T4 largely serves as a prohormone, T3 binds with high affinity to thyroid hormone nuclear receptors affecting gene transcription in target tissues and mediating its physiologic effects. It has positive inotropic and chronotropic effects in the heart and enhances myocardial adrenergic sensitivity. It also increases the rate of myocardial diastolic relaxation, augments intravascular volume, and lowers peripheral vascular resistance. It increases gastrointestinal motility, bone turnover, and regulates heat generation and energy expenditure.
Thyroid Examination
The thyroid gland is located in the neck midway between the sternal notch and thyroid cartilage. It attaches to the trachea posteriorly and elevates with swallowing. Examination involves both visualization and palpation while the patient swallows liquid. It can be palpated with the examiner behind the patient with circumferential hand positioning to allow focus on palpation or with the examiner facing the patient, which allows the examiner to see the thyroid during palpation. The anterior approach is preferred with necks of larger diameter.
Structural Disorders of the Thyroid Gland
Thyroid Nodules
Thyroid nodules are discrete structural lesions, distinct from the background gland parenchyma on ultrasound. They are most commonly detected as incidental findings on imaging studies performed for other reasons. The prevalence of nodules palpated on examination is 5% in women and 1% in men. They are detected on ultrasound in 40% of the U.S. population and are more common with increasing age. Thyroid nodules can result from multiple pathologic processes, ranging from benign cysts and inflammatory nodules, to malignancies including primary thyroid, lymphoma, or metastatic lesions. Non-thyroidal lesions, such as parathyroid adenomas, can also present as nodules. Primary thyroid neoplasms are clonal in origin and include follicular adenomas and thyroid cancer.
The initial evaluation of a thyroid nodule begins with measuring serum TSH (Figure 7). TSH suppression may indicate the presence of autonomously functioning or “hot” nodules, which account for 5% to 10% of palpable thyroid nodules. Autonomous nodules may cause hyperthyroidism and are associated with a very low risk of malignancy. They do not require fine-needle aspiration biopsy (FNAB). Patients with thyroid nodules and a suppressed TSH are evaluated with thyroid scintigraphy. A radioactive isotope, preferably iodine 123 (123I), is administered, the percentage taken up by the thyroid is calculated (radioactive iodine uptakem, RAIU), and an image is obtained (thyroid scan). Hot nodules concentrate radioactive iodine to a greater extent than normal thyroid tissue.
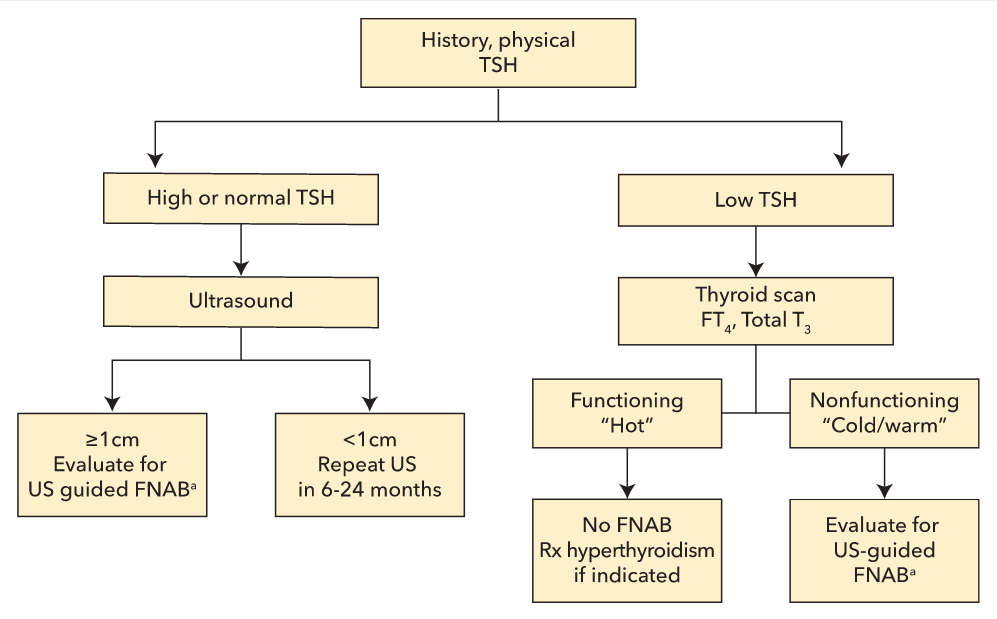 Initial evaluation of a thyroid nodule. There are size thresholds for FNAB based on US appearance. A less suspicious lesion may not need FNAB until it is larger than 2 cm, suspicious nodules if larger than 1 cm. FNAB = fine-needle aspiration biopsy; FT3 = free triiodothyronine; FT4 = free thyroxine; TSH = thyroid-stimulating hormone; US = ultrasound.
Initial evaluation of a thyroid nodule. There are size thresholds for FNAB based on US appearance. A less suspicious lesion may not need FNAB until it is larger than 2 cm, suspicious nodules if larger than 1 cm. FNAB = fine-needle aspiration biopsy; FT3 = free triiodothyronine; FT4 = free thyroxine; TSH = thyroid-stimulating hormone; US = ultrasound.
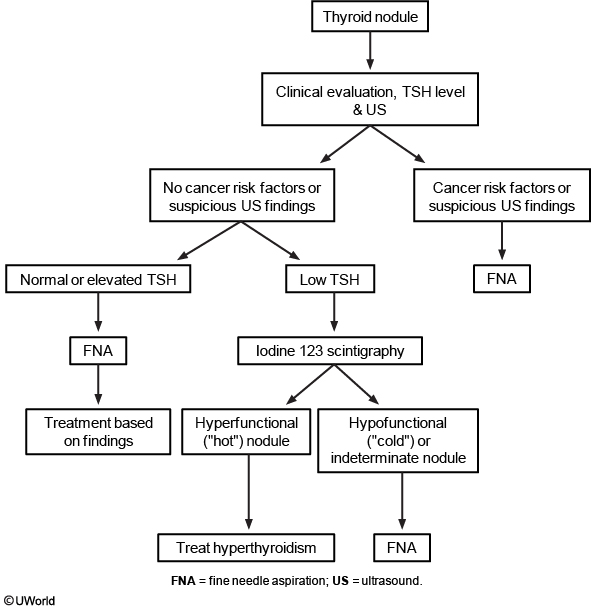
Thyroid ultrasonography with survey of the cervical lymph nodes should be performed in all patients with known or suspected thyroid nodules. This includes the subset of patients with low serum TSH levels who have undergone radionuclide thyroid scintigraphy suggesting nodularity, to evaluate both the presence of nodules concordant with the hyperfunctioning areas on the scan, which do not require FNAB, as well as other nonfunctioning nodules that meet sonographic criteria for FNAB. The management of nonfunctioning thyroid nodules is determined by the ultrasound results and presence of symptoms. The 2015 American Thyroid Association guidelines classify thyroid nodules into five sonographic patterns based on echogenicity, whether they are solid, cystic, or both, and features of malignancy (Table 32). Hyperechoic nodules are brighter, isoechoic nodules are equally bright, and hypoechoic nodules are darker than the background parenchyma. Ultrasound can determine the size of the nodule. FNAB is not recommended for subcentimeter nodules unless associated with symptoms, pathologic lymphadenopathy, or extrathyroidal extension.
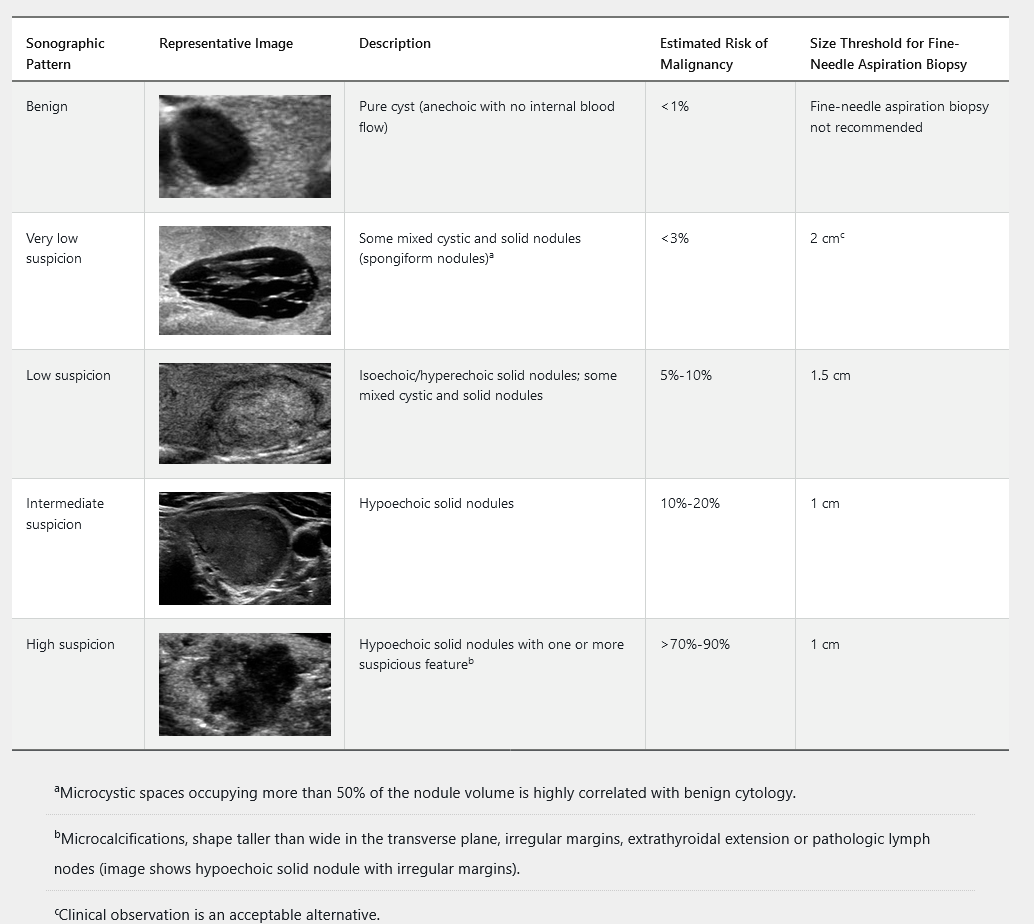
Thyroid nodule FNAB should be performed under ultrasound guidance when possible because of improved accuracy compared with palpation biopsy. Thyroid cytopathology is usually interpreted and classified according to criteria developed at the National Cancer Institute Thyroid Fine Needle Aspiration State of the Science Conference (Bethesda Conference). The Bethesda classification system is summarized in Table 33. Thyroid FNAB cytology can be nondiagnostic in up to 5% to 10% of specimens. Approximately 60% to 70% of biopsied nodules have benign cytology, 20% are indeterminate, and 5% to 10% have evidence of malignancy.
The management of cytologically indeterminate thyroid nodules (Bethesda III-V) can be challenging, and referral to an endocrinologist is recommended. Clinical monitoring is indicated for all thyroid nodules and should include measurement of serum TSH, as structurally abnormal thyroid glands are at increased risk for thyroid dysfunction (see Disorders of Thyroid Function). American Thyroid Association sonographic pattern and clinical context guide the timing of initial ultrasound follow up for benign nodules and those not evaluated with FNAB. Repeat ultrasound should be performed in 6 to 12 months for all high suspicion nodules, 12 to 24 months for intermediate and low suspicion nodules, and 24 months or longer for very low suspicion nodules. Repeat FNAB is indicated for all high suspicion nodules, nodules with concerning new sonographic findings, and intermediate or low suspicion nodules that increase significantly in size, which is defined as a 20% increase in at least two dimensions or an increase in nodule volume of more than 50%. Repeat FNAB is not recommended for nodules that have had two negative biopsies.
Goiters
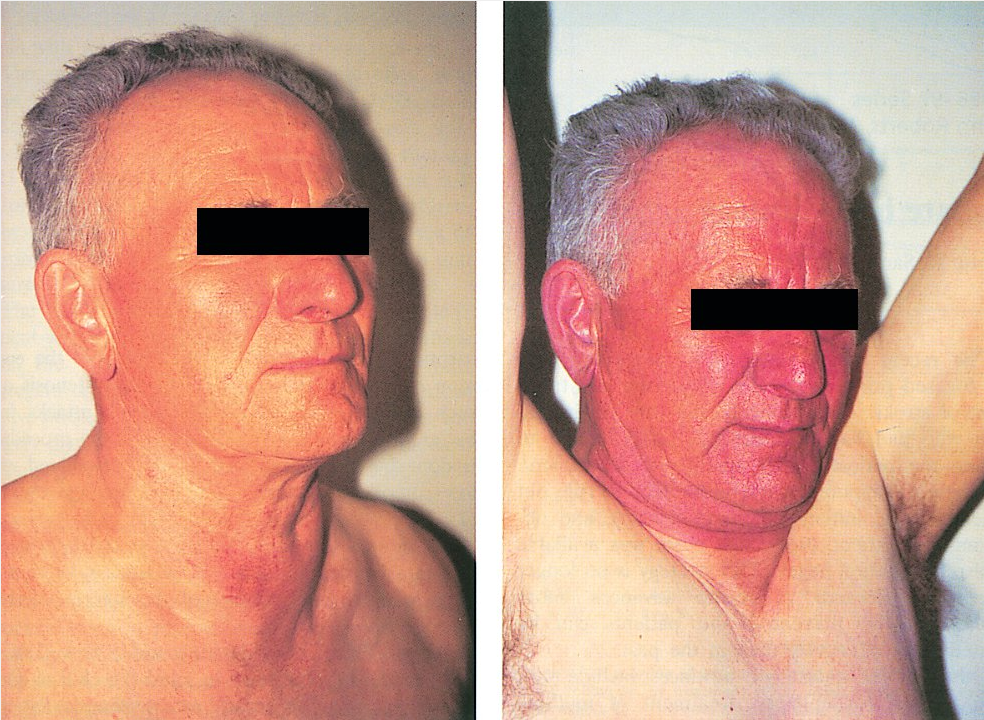
The term “goiter” denotes an enlarged thyroid gland. Goiters can be seen in the setting of normal thyroid function, hypothyroidism, or hyperthyroidism. The most common cause worldwide is endemic goiter due to severe iodine deficiency. Patients presenting with goiter should be questioned about iodine intake, rate of change in size, and risk factors for thyroid cancer (see Thyroid Cancer). Clinical history should focus on symptoms suggestive of thyroid hormone excess or deficiency and compression. Compressive symptoms include shortness of breath, cough, dysphagia, and voice changes and are evident in 10% to 20% of patients with goiter, most of whom also have clinically apparent thyromegaly. On examination tracheal deviation should be assessed and the size, symmetry, and consistency of the thyroid and presence of nodules should be noted. Possible venous obstruction should be assessed by having the patient raise the arms above the head. The findings of jugular venous distension, facial plethora, and flushing indicate possible thoracic outlet obstruction with reduced venous return (Pemberton sign) (Figure 8). Serum TSH level should be assessed in patients with goiter. If low, free T4 and total T3 should be measured and thyroid scintigraphy performed. If normal or elevated, thyroid/neck ultrasound is indicated in patients with risk factors for thyroid cancer, palpable thyroid nodules, gland asymmetry, large goiters, rapid growth pattern, or compressive symptoms. Patients with signs or symptoms of compression require additional testing as outlined below, and surgery may be needed for symptomatic management. Treatment of hypothyroid and hyperthyroid conditions in the setting of goiter is discussed below.
Multinodular Goiter
Multinodular goiter is the most common cause of goiter in older adults in the United States. Evaluation includes measurement of serum TSH and, when TSH is not suppressed, thyroid/neck ultrasound should be performed and discrete nodules evaluated as discussed previously (see Thyroid Nodules). The frequency of thyroid malignancy in patients with multinodular goiter is similar to those with solitary thyroid nodules. Signs and symptoms of compression or suspected substernal extension require additional testing. CT or MRI of the neck and chest (when substernal goiter is suspected) can define anatomic relationships and assess for tracheal narrowing. The administration of iodinated contrast should be avoided when possible to avoid precipitating iodine-induced hyperthyroidism (Jod-Basedow phenomenon). A flow-volume loop study is indicated in patients with symptoms of airway compression or when the tracheal lumen measures less than 1 cm in diameter on CT or MR (see MKSAP 18 Pulmonary and Critical Care Medicine). Endoscopy or a swallowing study can assess for extrinsic compression of the esophagus in patients with cervical dysphagia. Consultation with an otolaryngologist is indicated to confirm clinically suspected vocal cord paralysis. Surgery is indicated for significant compression or suspected malignancy.
Diffuse Goiter
The most common cause of diffuse goiter is autoimmune thyroid disease associated with thyroid dysfunction (Hashimoto thyroiditis and Graves disease). Infiltrative disorders, such as Riedel (IgG4-related) thyroiditis, are rare causes of diffuse goiter. Diffuse goiter may also occur in euthyroid patients in the absence of predisposing inflammatory or neoplastic processes. Genetic predisposition, iodine insufficiency, and cigarette smoking are contributing factors. Thyroid/neck ultrasound is indicated in euthyroid patients with diffuse goiter. It is recommended for patients with Graves disease or Hashimoto thyroiditis when there is thyroid gland asymmetry or nodules on examination. As discussed previously, additional testing is indicated if compressive signs or symptoms are present. Thyroid surgery is considered in the setting of significant compression.
Thyroid Cancer
Thyroid cancer is diagnosed in 13.9 per 100,000 people per year in the United States. The incidence of thyroid cancer has increased over the last four decades and now is the fifth most common cancer in women. Much of this change is attributable to a rise in the diagnosis of small noninvasive cancers, initially detected incidentally on imaging done for other reasons (carotid Doppler studies, neck/chest CT, PET scan). Mortality rates have remained stable with an overall 5-year survival rate of 98.1%. Papillary thyroid carcinoma and follicular thyroid carcinoma, collectively known as differentiated thyroid cancer, account for the most thyroid cancer diagnoses in the United States. Papillary thyroid carcinoma commonly spreads to cervical lymph nodes but is associated with a low risk of distant metastases; whereas lymph node metastases are rare in follicular thyroid carcinoma, metastases to lung, bone, and other sites can be seen. The types and frequency of thyroid cancer are shown in Figure 9.
Thyroid cancer is often identified incidentally; however, it may be detected on neck examination. Risk factors for thyroid cancer include a personal history of ionizing radiation exposure with a higher prevalence of papillary thyroid carcinoma in persons exposed to ionizing radiation (>10 rads), with the highest risk seen following childhood exposures, such as with nuclear accidents (Chernobyl), and a personal or family history of thyroid malignancy. Additional risk factors for thyroid cancer include extremes of age (younger than 30 or older than 60) and male gender. Findings suggestive of malignancy include rapid nodule growth, a hard fixed nodule, dysphagia, vocal cord paralysis (hoarseness), and cervical lymphadenopathy. The diagnosis is confirmed by fine-needle aspiration biopsy.
Surgery is the mainstay of thyroid cancer treatment. Either total thyroidectomy or hemithyroidectomy is acceptable for unilateral differentiated thyroid cancers measuring 1 to 4 cm, as long as locoregional spread is not suspected. Total thyroidectomy is otherwise indicated. Unique risks of thyroid surgery include hypocalcemia as a result of removal or devascularization of the parathyroid glands and difficulty breathing or voice changes from recurrent laryngeal nerve injury. Referral to a high-volume thyroid surgeon (>90 cases per year) is preferred due to a lower risk of postoperative complications.
Postoperative radioactive iodine (131I) should be considered for the dual purposes of thyroid remnant ablation and adjuvant therapy for patients with differentiated thyroid cancer and an intermediate to high risk of recurrence, such as with extrathyroidal extension, lymphovascular invasion, poorly differentiated/more aggressive histology, or metastatic disease. TSH stimulation, achieved either by withdrawal of thyroid hormone (levothyroxine) replacement or administration of recombinant human TSH (rhTSH), is required to promote 131I uptake by thyroid follicular cells. Following 131I therapy, patients undergo whole-body scanning to identify areas of 131I uptake corresponding to metastatic disease. Uptake is expected in the postsurgical thyroid bed but not elsewhere. 131I therapy is also used to treat thyroid cancer recurrences not amenable to surgical resection.
After initial cancer treatment, serum thyroglobulin (Tg), a sensitive marker for the detection of persistent or recurrent disease, and thyroglobulin antibody (TgAb) titers are monitored. When TgAb is present, Tg levels are uninterpretable because TgAb can falsely lower Tg measurement. In this case, the TgAb level serves as a surrogate marker. A falling TgAb titer over time correlates with a favorable prognosis, whereas a rising titer is suspicious for persistent or recurrent disease.
Neck ultrasound is regularly performed in routine thyroid cancer surveillance, usually 6 to 12 months after the initial cancer treatment. In patients at high risk of recurrent disease, diagnostic radioactive iodine (123I or 131I) whole-body scanning with TSH-stimulated Tg measurement can be performed. If residual or recurrent thyroid cancer is suspected, such as when serum Tg is persistently elevated or rising over time but not identified by neck ultrasound or radioactive iodine whole-body scanning, adjunctive imaging tests including CT, MRI, bone scan, or PET/CT can be useful in disease localization.
Treatment of intermediate to high-risk differentiated thyroid cancer includes TSH suppression with daily levothyroxine. Thyroid follicular cells are TSH responsive, as are most well-differentiated thyroid cancers. To reduce cancer recurrence, a sufficient dose of levothyroxine is administered to suppress the serum TSH below normal with the specific goal individualized. A serum TSH level less than or equal to 2 but at or above the lower limit of the reference range can be targeted in patients with low risk thyroid cancer. Monitoring and dose adjustment by internists is appropriate in conjunction with the endocrinologist. Metastatic thyroid cancer is managed with active surveillance, additional surgery, or 131I therapy, followed by external beam radiation therapy and/or chemotherapy (tyrosine kinase inhibitors).
Anaplastic thyroid cancer is a rare but aggressive thyroid malignancy that can occur in patients with preexisting differentiated thyroid cancer or de novo. It carries a dismal prognosis with median survival of 5 months. Anaplastic thyroid cancer presents with a rapidly enlarging neck mass and may be unresectable at the time of diagnosis. Treatment is palliative in most cases with surgery, external beam radiation therapy, and chemotherapy.
Medullary thyroid cancer arises from parafollicular cells. Germline RET oncogene mutations occur with familial medullary thyroid cancer and multiple endocrine neoplasia (MEN) 2A and 2B. MEN should be ruled out with genetic testing prior to surgery, given its association with pheochromocytoma. Medullary thyroid cancer is treated with total thyroidectomy and central neck lymph node dissection. Levothyroxine is indicated to treat postoperative hypothyroidism in patients with medullary thyroid cancer with a goal serum TSH level within the reference range. Serum calcitonin, serum carcinoembryonic antigen levels, and neck ultrasound are part of routine cancer surveillance.
Low-risk papillary thyroid cancer, confined to the thyroid gland, that has been completely resected, has not metastasized, and does not demonstrate aggressive pathologic features (lymphovascular invasion or tall cell variant), requires no additional treatment. The risk of disease-related death is less than 1%, and the risk of structural disease recurrence is 1% to 2% for low-risk unifocal papillary microcarcinomas. Patients receiving either lobectomy or thyroidectomy have similarly excellent outcomes.
Evaluation of Thyroid Function
Serum TSH is the most sensitive and recommended initial test of thyroid function. It is used to determine euthyroidism, hypothyroidism or hyperthyroidism. If TSH is suppressed, free T4 and total T3 should be assessed to detect overt or subclinical hyperthyroidism, and if TSH is elevated, free T4 should be assessed to detect overt or subclinical hypothyroidism. Measuring serum TSH alone is sufficient except in certain circumstances, such as suspected central hypothyroidism, where free T4 measurement is also indicated (see Disorders of the Pituitary Gland).
Total and free T4 and total T3 concentrations can be assessed with a variety of assays and can be accurately measured in most patients with overt thyroid dysfunction. Commercially available free T3 assays are less reliable. Perturbations in thyroxine-binding globulin and other binding proteins can occur with physiologic changes (pregnancy), certain disease states (nephrotic syndrome), and as a result of medications (oral estrogen therapy). Levels of total T4 and T3 will vary based on increasing or decreasing binding proteins and do not reflect actual thyroid function. Measurement of free T4, the unbound fraction of T4 in serum, is most commonly determined using widely available immunometric assays. These tests are accurate in most clinical settings, including in patients with mild binding protein derangements; however, they can be inaccurate with more significant perturbations (familial dysalbuminemic hyperthyroxinemia). Measuring free T4 by equilibrium dialysis is highly accurate, but expensive, not widely available, and rarely necessary.
Patients taking more than 5 to 10 mg/day of biotin should be counseled to discontinue ingestion for 2 days prior to the laboratory assessment of thyroid function. Biotin is a water-soluble vitamin that is commonly found in over-the-counter dietary supplements. High circulating levels of biotin have been shown to interfere with laboratory assays that utilize streptavidin–biotin as an immobilizing system. Biotin interference causes falsely high results with competitive immunoassays used to measure small molecules (free T4, free T3, total T4, and total T3) and causes falsely low results with sandwich assays used to measure large molecules (TSH).
Measurement of T3 is recommended in three settings: (1) in the evaluation of thyrotoxicosis to identify isolated T3 toxicosis, (2) to assess the severity of hyperthyroidism and response to therapy, and (3) potentially, to differentiate hyperthyroidism from destructive thyroiditis. In T3 toxicosis, the T3:T4 ratio is often greater than 20 due to preferential secretion of T3. Multiple drugs can affect thyroid function and replacement (Table 34).
Measurement of T3 in the setting of hypothyroidism is not necessary or recommended; normal levels are maintained unless hypothyroidism is severe. TSH will become elevated in hypothyroidism first, followed by abnormalities in T4 level.
There is no clinical indication to assess reverse T3 levels, and thus it is not recommended.
T3 is produced primarily by deiodination of T4 in peripheral tissues. Serum T3 levels (total and free) correlate poorly with overall thyroid physiologic status and are less reliable than T4, although they are occasionally used in the evaluation of hyperthyroidism in patients with normal T4 levels, in those with alterations in thyroid-binding proteins, or in those with euthyroid sick syndrome. T3 has a shorter half-life than T4, and liothyronine (T3) therapy can lead to fluctuations in physiologic status and increased risk of thyrotoxicosis. Studies have not shown superiority of combined replacement over levothyroxine (T4) alone.
Disorders of Thyroid Function
Thyroid Hormone Excess (Hyperthyroidism and Thyrotoxicosis)
The term thyrotoxicosis describes the exposure of tissues to high levels of circulating thyroid hormones (T4 and/or T3) from any cause. Hyperthyroidism is thyrotoxicosis caused by excessive endogenous production of thyroid hormones. The overall prevalence of hyperthyroidism in the United States is 1.3%. In primary hyperthyroidism, the thyroid gland is the anatomic site of dysfunction. Increased secretion of TSH is a rare secondary cause of hyperthyroidism.
Clinical Features and Diagnosis
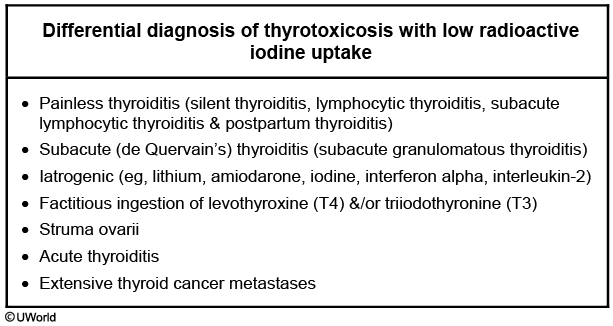
Table 35 lists signs and symptoms of thyroid hormone excess. In elderly patients hyperthyroidism may be apathetic instead of presenting with classic symptoms. Lid lag (eyelid retraction) can be seen in thyrotoxicosis of any cause and results from increased adrenergic tone. The diagnosis of hyperthyroidism is based on biochemical testing demonstrating a low-serum TSH level and elevated concentrations of free T4 and/or total T3. Thyroid scintigraphy with radioactive iodine uptake (RAIU) can verify the cause. RAIU is high (above 30%) or inappropriately normal in hyperthyroidism and low (less than 10%) in other causes of thyrotoxicosis.
Additional testing can be done when the clinical diagnosis is not clear; when RAIU is unavailable or unreliable (patients on amiodarone, lithium, or exposed to recent iodinated contrast material); or when scintigraphy is contraindicated (pregnancy and lactation). Tests include measurement of thyroid-stimulating immunoglobulin (TSI) or thyrotropin (TSH) receptor antibodies (TRAb) if Graves disease is suspected but the diagnosis remains clinically unclear, and thyroid ultrasound to assess for patterns of vascularity.
Causes
Causes of thyrotoxicosis are listed in Table 36. Graves disease, toxic multinodular goiter, and toxic adenoma are the most common causes of hyperthyroidism.
Graves Disease
Graves disease is a multisystem disease and can affect the thyroid, ocular muscles, and skin. It causes 80% of hyperthyroidism in iodine-sufficient areas. It is an autoimmune thyroid disorder predominantly affecting women with a peak incidence among patients aged 30 to 60 years. Graves disease is also more common in patients with other autoimmune disorders or a family history of thyroid autoimmunity. T lymphocytes become sensitized to thyroid antigens and stimulate B lymphocytes to produce antibodies against the TSH receptor (TSI or TRAb). The thyroid is diffusely enlarged, may have a bruit, and has a firm, smooth texture on examination; cervical lymphadenopathy can occur. Systolic hypertension, tachycardia, hyperreflexia, and warm moist skin are often present.
Graves ophthalmopathy affects 25% of patients. Cigarette smoking is a risk factor. Clinical manifestations include periorbital edema, chemosis (conjunctival edema), proptosis (protrusion of the globe), diplopia (due to oculomotor paresis), and vision loss. Graves ophthalmopathy does not respond to the treatment of hyperthyroidism and often requires glucocorticoids or surgery; teprotumumab, a monoclonal antibody to insulin-like growth factor I receptor, is an option for moderate-to-severe disease in patients unresponsive to or intolerant of glucocorticoids.
Pretibial myxedema is a rare infiltrative dermopathy of Graves disease that affects 2% to 3% of patients; it is a nonpitting edema that is indurated, with a peau d’orange appearance, typically on the shins (see MKSAP 18 Dermatology).
Toxic Adenoma and Multinodular Goiter
Toxic adenoma and multinodular goiter typically affect older adults as the prevalence of thyroid nodules increases with age. Thyroid nodules synthesize and secrete thyroid hormones independent of TSH stimulation. Exposure to iodinated contrast can precipitate conversion from nontoxic to toxic adenoma(s), such as with contrasted CT scanning and cardiac catheterization.
Destructive Thyroiditis
Thyrotoxicosis occurs in destructive thyroiditis as a result of unregulated release of preformed thyroid hormone from thyroid follicles damaged by inflammation. Causes are listed in Table 37. Thyroiditis typically has three phases: thyrotoxic, hypothyroid, and return to euthyroidism. The first two phases can last up to 3 months each. A person has increased risk of additional bouts of thyroiditis once the initial thyroiditis has resolved.
Exogenous
This patient presents with thyrotoxicosis that is most likely due to exogenous thyroid supplements. Obese patients often attempt to lose weight by taking dietary supplements that may contain high T3 amounts. Patients present with typical hyperthyroid symptoms (e.g., weight loss, palpitations, etc.), but do not have exophthalmos as seen in Graves’ disease. However, patients may have lid lag. There is usually no goiter on examination, since the exogenous thyroid hormones inhibit TSH secretion and cause thyroid atrophy.
Laboratory studies usually show high T3, low serum thyroglobulin levels, and suppressed TSH. Radioactive iodine uptake scan shows < 1% uptake (due to suppressed TSH). Serum thyroglobulin is low in exogenous thyroid hormone-induced thyrotoxicosis, but normal to elevated in all other causes of thyrotoxicosis. Fecal measurement of thyroid hormones has also been used to diagnose factitious thyrotoxicosis.
De Quervain Thyroiditis
This patient’s presentation of fever, anterior neck pain, tender thyroid gland, and symptoms of thyrotoxicosis are suggestive of subacute granulomatous thyroiditis, also known as de Quervain thyroiditis. These patients usually have a preceding viral infection prior to development of the postviral inflammatory process.
Neck pain is the dominant presenting symptom in most patients, and pain can radiate to the jaw. Fatigue, malaise, anorexia, and fever may also be present. The thyroid may be enlarged but is almost always tender to palpation. Patients usually follow a predictable clinical course of hyperthyroidism, followed by euthyroidism, hypothyroidism, and thyroid function recovery. Each phase can last up to 8 weeks. However, some patients may have only the hyperthyroid or hypothyroid phase. ESR and CRP are elevated. Radioactive iodide uptake scan done during the hyperthyroid phase shows low uptake.
Treatment of subacute thyroiditis involves symptomatic pain relief. High-dose nonsteroidal anti-inflammatory drugs (NSAIDs) (eg, ibuprofen 1200-3200 mg/day) are usually helpful. If pain is not relieved in 2-3 days with NSAIDs, they should be discontinued and treatment with prednisone (40 mg/day) should be initiated. Prednisone can also be used as initial therapy for patients with severe pain.
Thyrotoxicosis is present in about 50% of cases in the early phases of the disease. These symptoms are best managed with beta blockers. However, thionamides and radioactive iodine therapy (Choices C and E) are not useful as symptoms are due to the release of preformed thyroid hormones. Most individuals do not need treatment for the hypothyroid phase of subacute thyroiditis. Thyroid function tests should be measured every 2-8 weeks in all patients. About 95% of patients recover completely, and only 5% develop permanent hypothyroidism.
Management
Most thyrotoxic patients benefit from β-blockers (atenolol, metoprolol, propranolol) to reduce adrenergic symptoms rapidly. Propranolol decreases the peripheral conversion of T4 to T3, but is non-cardioselective and requires twice or three times daily dosing. Atenolol and metoprolol are preferred owing to once-daily dosing that increases adherence and their cardioselective nature that decreases central nervous system side effects.
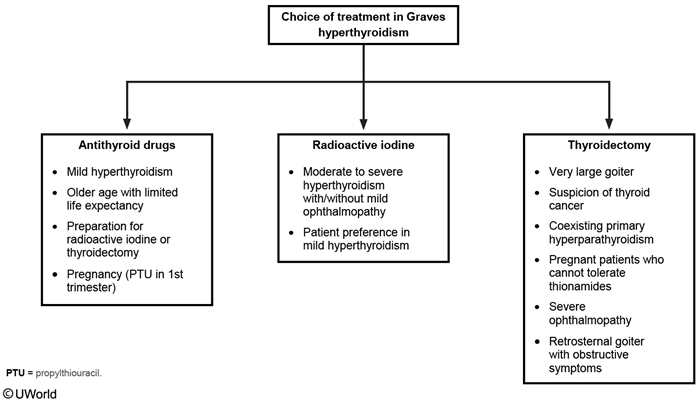
There are three treatment modalities for hyperthyroidism: thioamides (methimazole and propylthiouracil [PTU]), radioactive iodine (131I) ablative therapy, and thyroidectomy. The choice of treatment is predicated on the cause of the hyperthyroidism and patient preference. Short-term use of methimazole to normalize thyroid function prior to 131I therapy or thyroidectomy is recommended for patients 65 years of age or older or who have prevalent cardiovascular disease or multiple comorbidities. Referral to an endocrinologist is recommended.
Graves Disease
Antithyroid drugs (thionamides) are often used in the initial treatment of Graves hyperthyroidism because up to 50% will have spontaneous remission of hyperthyroidism within 24 months, especially if the goiter is small and only low doses of thioamide are required to achieve euthyroidism. Recurrent hyperthyroidism is likely if TRAb levels remain elevated at the time of drug discontinuation. If Graves hyperthyroidism recurs, definitive treatment is recommended.
Agranulocytosis and liver dysfunction are two rare but serious side effects of thioamides. Prior to treatment, baseline CBC with differential and liver profile should be assessed. Agranulocytosis should be suspected and the patient's neutrophil count assessed in the setting of fever or pharyngitis. Liver function should be assessed in any patient with symptoms or signs of hepatic dysfunction (jaundice, icterus). Methimazole is the antithyroid drug of choice, except in the first trimester of pregnancy, because PTU has been associated with fatal hepatonecrosis.
The goal of 131I ablative therapy in Graves disease is to render the patient hypothyroid. Women receiving 131I therapy must avoid pregnancy for 6 to 12 months after treatment. In patients with Graves ophthalmopathy, there is an acute escalation of thyroid autoantibody titers following radioiodine therapy that may exacerbate ocular symptoms. Pretreatment of Graves ophthalmopathy or selection of alternative treatments depends on the severity of the eye disease.
Thyroidectomy in Graves hyperthyroidism is most appropriate when there is a large goiter with compressive symptoms, moderate to severe Graves ophthalmopathy, and/or with coexistent thyroid cancer or primary hyperparathyroidism.
Other Causes
First-line therapy for a toxic adenoma or toxic multinodular goiter is either 131I therapy or thyroid surgery. Choice is determined by patient preference, presence of compressive symptoms, and access to a high-volume thyroid surgeon.
Destructive thyroiditis is managed expectantly with β-blockers to control adrenergic symptoms and NSAIDs, followed by high-dose glucocorticoid therapy, for pain control in painful thyroiditis.
Subclinical Hyperthyroidism
Subclinical hyperthyroidism diagnosis is based on suppression of the serum TSH, with normal T4 and T3 levels. Subclinical hyperthyroidism affects 0.7% of the U.S. population. Approximately 0.5% to 7% progress to overt hyperthyroidism per year and 5% to 12% revert to normal thyroid function. The most common cause is toxic multinodular goiter.
Subclinical hyperthyroidism has been associated with an increased risk of atrial fibrillation and cardiovascular events. A recent large prospective cohort study demonstrated higher rates of hip fracture with subclinical hyperthyroidism (7% in subclinical hyperthyroidism vs. 4.5% in euthyroid patients); however, it is unknown whether treatment reduces fracture risk.
The TSH will normalize at 6 weeks in more than 25% of patients with subclinical hyperthyroidism. Therefore, observation and rechecking thyroid function prior to the initiation of treatment is reasonable unless the risk of complications is high, such as in patients with cardiac disease. Higher risk of cardiovascular and skeletal complications is seen with serum TSH level under 0.1 µU/mL (0.1 mU/L). Treatment of subclinical hyperthyroidism is recommended for patients with serum TSH levels below 0.1 µU/mL (0.1 mU/L) and with symptoms, cardiac risk factors, heart disease, or osteoporosis, as well as for postmenopausal women not taking estrogen therapy or bisphosphonates.
Thyroid Hormone Deficiency
Thyroid hormone deficiency affects more than 10 million Americans. It is 10 times more common in women than men.
Clinical Features and Diagnosis
Signs and symptoms of thyroid hormone deficiency are listed in Table 35. Thyroid hormone deficiency is also associated with laboratory abnormalities including anemia, elevated LDL cholesterol, and hyponatremia.
The diagnosis of primary hypothyroidism is made by measuring serum TSH, and if elevated, measuring free T4, which can be added on in most laboratories. Serum TSH is elevated in both overt and subclinical hypothyroidism, but free T4 is normal when subclinical and low in overt hypothyroidism. Thyroid autoantibodies [thyroid peroxidase (TPO) antibodies] are present in most patients with Hashimoto thyroiditis; however, measurement of TPO antibody titer is not necessary unless the diagnosis is unclear. The diagnosis of hypothyroidism is discussed elsewhere (see Disorders of the Pituitary Gland).
Causes
Causes of hypothyroidism are listed in Table 38. The most common cause in the United States is autoimmune thyroid gland failure (due to Hashimoto thyroiditis), while iodine deficiency, which affects 2 billion people worldwide, is the most common cause globally. Iodide deficiency is uncommon in the United States as a result of efforts to fortify food (iodized salt). Central hypothyroidism is also uncommon.
Primary Hypothyroidism
Hashimoto thyroiditis (chronic lymphocytic thyroiditis) is an autoimmune thyroid disorder characterized by diffuse infiltration of the thyroid gland by lymphocytes and plasma cells with subsequent follicular atrophy and scarring. It is more common in patients with other autoimmune disorders or a family history of thyroid autoimmunity. Diffuse goiter can be seen most commonly in younger patients. Most patients (90%) have TPO antibodies, and the risk of developing hypothyroidism is four times higher in euthyroid patients with TPO antibodies.
Hypothyroidism occurs in all patients after thyroidectomy and 20% of patients after thyroid lobectomy. Postablative hypothyroidism occurs after 131I therapy in 90% of patients with Graves disease within 1 year of radioactive iodine therapy, and in 60% of patients with toxic multinodular goiter, although onset of hypothyroidism may be delayed for many years in the latter case. External beam radiation to the neck also can cause hypothyroidism, as with Hodgkin lymphoma and head/neck malignancies.
Drug-induced hypothyroidism is another cause of primary hypothyroidism (see Table 38).
Subclinical Hypothyroidism
Subclinical hypothyroidism is typically asymptomatic and diagnosed by a serum TSH level above the upper limit of the reference range and a normal free T4 level. It affects 5% to 10% of the general population. Transient elevation of serum TSH should be ruled out by repeating the measurement in 6 to 8 weeks. The rate of progression from subclinical to overt hypothyroidism is 2% to 4% per year, while one-third of patients spontaneously revert to normal thyroid function. The normal range for TSH increases with age, and a TSH level of up to 10 µU/mL (10 mU/L) is within the normal range for persons 80 years of age and older.
Subclinical hypothyroidism with TSH above 10 µU/mL (10 mU/L) may be a risk factor for coronary artery disease and heart failure. There is no evidence that treating subclinical hypothyroidism improves quality of life, cognitive function, blood pressure, or weight, but in patients with elevated LDL cholesterol, normalization of the TSH will lower LDL cholesterol.
Management
Thyroid hormone replacement with levothyroxine is the treatment of choice for thyroid hormone deficiency. Goals of treatment are to normalize serum TSH (in primary hypothyroidism) or free T4 (in central hypothyroidism) and to resolve signs and symptoms of hypothyroidism. Beginning a full replacement dose (1.6 µg/kg lean body weight) is appropriate for most patients with overt hypothyroidism, except in older adults and patients with cardiovascular disease for whom lower initial doses (25-50 µg/day) are recommended. Assessment of the adequacy of treatment should be done with a repeat serum TSH level at least 6 weeks after initiation or change in dose with a goal of a normal TSH.
T3-containing compounds are not recommended in the treatment of hypothyroidism due to its short half-life, which results in spikes in T3 levels. Studies have failed to show that T3 alone or in combination with T4 has clear benefit in treatment of hypothyroidism.
Subclinical hypothyroidism with a serum TSH value above 20 µU/mL (20 mU/L) should be treated. Initial treatment is 25 to 50 µg of levothyroxine per day. There is unclear benefit and potential for harm in treating TSH values in the range of 5 to 20 µU/mL (5-20 mU/L]). A recent study showed no benefit to treatment of subclinical hypothyroidism in patients over the age of 65 years with TSH levels between 4.60 µU/mL (4.60 mU/L) and 19.99 µU/mL (19.99 mU/L), although outcomes were assessed at 2 years, potentially before cardiovascular benefits could emerge. Overtreatment, however, is seen in more than one-third of patients over age 65 years, which may increase risk for dysrhythmia and bone loss. Treatment for subclinical hypothyroidism with TSH less than 20 µU/mL (20 mU/L) should be considered in younger patients, those attempting to become pregnant, or if severe symptoms are present.
Oral levothyroxine is absorbed in the jejunum and ileum. Ideally it should be taken on an empty stomach (60 minutes before breakfast or coffee are consumed). If the patient is having difficulty adhering to morning administration, then it can be taken before bed. Missed doses can be taken the following day in younger patients struggling with daily adherence. The absorption of an orally administered dose is 70% to 80% under optimum fasting conditions. Gastrointestinal disorders (such as celiac disease) may impact absorption and result in higher than expected levothyroxine dose requirements. Medications can also interfere with the absorption or metabolism of levothyroxine (see Table 34).
Drug-Induced Thyroid Dysfunction
Many medications can affect thyroid function and are listed in Table 34.
Amiodarone is an antiarrhythmic medication with high iodine content (37%) and prolonged half-life of approximately 60 days. Thyroid dysfunction occurs in approximately 25% of amiodarone-treated patients and can present as hypothyroidism, hyperthyroidism, painless thyroiditis, or goiter. Hypothyroidism, seen in 20% of affected patients, is usually seen in the setting of Hashimoto thyroiditis. All patients can have a transient rise in TSH levels in the first few months of amiodarone therapy due to the Wolff-Chaikoff effect (temporary decrease in thyroid production due to iodine load), but most regain normal thyroid function.
Thyrotoxicosis affects 5% of patients treated with amiodarone. Type 1 (hyperthyroidism) amiodarone-induced thyrotoxicosis occurs in patients with Graves disease or thyroid nodules. It is a form of iodine-induced hyperthyroidism (Jod-Basedow phenomenon). It is treated with thioamides, typically methimazole. Type 2 (destructive thyroiditis) amiodarone-induced thyrotoxicosis is more common and occurs in patients without underlying thyroid disease. It is usually self-limiting but sometimes requires treatment with moderate- to high-dose glucocorticoids. The decision to discontinue amiodarone depends on the patient's cardiac condition and type of thyrotoxicosis (type 1 or 2).
Thyroid Function and Dysfunction in Pregnancy
Thyroid hormones are essential for normal fetal development. The size of the maternal thyroid gland can increase up to 40% during pregnancy. Production of T4 and T3 increases up to 50% to compensate for the increased thyroxine-binding globulin production associated with pregnancy-related increase in estrogen. Iodine requirements also increase up to 50%. Pregnant and lactating women should therefore be counseled to supplement their dietary iodine intake with a daily oral supplement containing 150 µg of iodine, which is included in some but not all over-the-counter and prescription prenatal vitamins. Universal screening of TSH in pregnant women is not recommended. However, those at increased risk of thyroid dysfunction should be screened, which includes those 30 years of age and older; with known hypothyroidism and/or a strong family history of thyroid dysfunction; prior head/neck irradiation; prior neck surgery; positive TPO, TSI, or TRAb status; or other autoimmune disorders.
Changes in thyroid function tests are depicted in Figure 10. Placental human chorionic gonadotropin (hCG) stimulates thyroid hormone secretion and TSH may be mildly suppressed as a result. In the late first trimester (weeks 7-12) the lower limit of the TSH reference range decreases by 0.4 µU/mL (0.4 mU/L) and upper limit by 0.5 µU/mL (0.5 mU/L). Serum TSH gradually returns to the nonpregnant reference range in the second and third trimester.
Measured total T4 concentrations increase linearly during pregnancy. After week 16, the upper limit of the total T4 reference range can be estimated by multiplying the nonpregnant upper limit by 1.5. Free T4 measured by indirect analogue immunoassays are inaccurate in pregnancy unless method and trimester-specific reference ranges are applied.
Consultation with an endocrinologist is indicated for management of thyrotoxicosis during pregnancy. Gestational thyrotoxicosis from high human chorionic gonadotropin levels is the most common cause of transient TSH suppression. If serum total or free T4 remains within the trimester-specific reference range, treatment is not needed. Women with moderate to severe hyperthyroidism in early pregnancy should be treated with PTU because potential teratogenic effects are less severe than with methimazole. After the first trimester, women can be transitioned to methimazole. Thyroid function should be followed closely and the serum total or free T4 should be maintained at or just above the trimester-specific reference range to avoid fetal hypothyroidism.
Graves disease affects 0.2% of pregnant women and can be confirmed by classic physical findings or elevated TSI or TRAb. Women with Graves disease are considered high-risk pregnancies and should be followed by maternal fetal specialists throughout the pregnancy.
Hypothyroidism in pregnancy is associated with increases in miscarriage, premature birth, low birth weight, and decreased infant neurocognitive function. Levothyroxine is the treatment of choice.
For women with preexisting hypothyroidism, levothyroxine dosing can be empirically increased by 30% when pregnancy is confirmed. In treatment-naïve pregnant women with positive TPO antibodies, levothyroxine is started if TSH level is ≥2.5 µU/mL (2.5 mU/L). Treatment is indicated for TPO-negative pregnant women if TSH is above the pregnancy-specific reference range. TSH should be measured every 4 weeks for the first half of pregnancy and around 30 weeks in all hypothyroid women and in those at risk for hypothyroidism (antibody positive or history of hemithyroidectomy or 131I therapy). A TSH level less than 2.5 µU/mL (2.5 mU/L) should be targeted in treated hypothyroid women both preconception and during pregnancy.
Thyroid nodules detected in pregnant women should be evaluated as in nonpregnant patients. The timing of FNAB, whether during or after pregnancy, is determined by the likelihood of cancer and patient preference. Consultation with an endocrinologist is indicated for management of thyroid cancer detected during pregnancy. Pregnant women with a history of thyroid cancer should be managed as when not pregnant.
Nonthyroidal Illness Syndrome (Euthyroid Sick Syndrome)
Nonthyroidal illness syndrome (NTIS) commonly occurs in patients who are hospitalized and critically ill. Up to 75% of hospitalized patients have thyroid function test abnormalities. Nonthyroidal illness suppresses thyrotropin-releasing hormone, which typically results in suppressed but detectable TSH. An undetectable TSH is not consistent with NTIS. Infrequently, TSH can be mildly elevated in NTIS, but a TSH level of 20 µU/mL (20 mU/L) or greater is not consistent with NTIS. T4 is typically normal, but due to decreased deiodinase activity in T4 metabolism, T3 decreases and reverse T3 increases (biologically inactive). Thyroid-binding globulin decreases in illness, lowering the total T4 and T3 levels. NTIS can be interpreted as an adaptive response to systemic illness and macronutrient restriction.
Treatment of NTIS is not recommended due to lack of significant clinical benefit. In general, thyroid function should not be assessed in hospitalized patients unless there is a strong clinical suspicion of thyroid dysfunction. If NTIS is diagnosed, TSH should be rechecked approximately 6 weeks after the patient has recovered from their nonthyroidal illness to assess for return to normal.
Thyroid Emergencies
Thyroid Storm
Thyroid storm is a rare disorder with high mortality (up to 30%) characterized by severe thyrotoxicosis (suppressed TSH, elevated free T4 and/or total T3) and systemic hemodynamic decompensation (shock). Serum thyroid hormone concentrations do not differentiate thyroid storm from severe thyrotoxicosis. It is the presence of shock that makes the diagnosis of thyroid storm.
Presentation often follows discontinuation of antithyroid drug therapy, systemic illness, labor and delivery, surgery, or trauma. Patients with Graves disease are at higher risk. Clinical manifestations include high fever, tachycardia, altered mental status, and cardiac and hepatic dysfunction. A scoring system, such as the Burch and Wartofsky Point Scale (Table 39), can support the diagnosis, but thyroid storm is diagnosed clinically.
Management includes ICU-level care, treatment of any precipitant illness, thyrotoxicosis-directed therapy, and supportive measures. Thyrotoxicosis is treated with intravenous β-blockers (esmolol infusion), thioamide (typically PTU, transitioning to methimazole when more stable), intravenous high-dose glucocorticoids, and potassium iodide. Iodide should be administered more than 1 hour after antithyroid drugs to avoid providing substrate to the gland. Glucocorticoid therapy is a potent inhibitor of peripheral T4 to T3 conversion. Bile acid sequestrants can be used to decrease T4 and T3 levels, especially in patients unable to take thioamides. Plasmapheresis or emergent thyroidectomy is used in patients who respond poorly to medical therapy. Definitive treatment with thyroidectomy or 131I therapy is indicated in patients who survive thyroid storm.
Myxedema Coma
Myxedema coma is a life-threatening presentation of severe hypothyroidism with hemodynamic compromise that affects 0.22 people per million per year. Mortality is high (up to 40%), and ICU-level care is required. Risk factors for myxedema coma are female gender, advanced age, cold exposure, or a precipitant event in patients with undiagnosed hypothyroidism, such as myocardial infarction, sepsis, trauma, or stroke. Mental status changes ranging from lethargy to coma to psychosis, coupled with hypothermia (temperature below 34.4 °C (94.0 °F) are the most common clinical manifestations. Bradycardia, hypotension, or decreased respiration rate with resultant hypoxia and hypercapnia are also frequently present. Careful examination of the neck for thyroidectomy scar is critical. Free T4 is low in myxedema coma. TSH is typically elevated, but without an overtly low free T4, myxedema coma is unlikely regardless of how high the TSH. Other metabolic derangements include hyponatremia and hypoglycemia. Cortisol should be drawn with initial laboratory studies to assess for concomitant cortisol deficiency.
Aggressive supportive measures include fluids, vasopressors if necessary, ventilator support, and passive warming rather than active warming to avoid vasodilation, which can worsen hypotension. Stress-dose glucocorticoids (100 mg intravenous hydrocortisone every 8 hours) are administered empirically before thyroid hormone is initiated to treat possible concomitant adrenal insufficiency. If random cortisol level is above 18 µg/dL (496.8 nmol/L), hydrocortisone can be discontinued. Replacement of thyroid hormone requires consideration of the need to normalize the thyroid hormone level rapidly and the risk of a fatal cardiac event caused by thyroid hormone administration. Initial treatment is intravenous levothyroxine with loading dose of 200 to 400 µg, followed by a daily oral dose of 1.6 µg/kg. The dose should be reduced to 75% if administered intravenously. Lower levothyroxine doses are recommended with advanced age and/or cardiac disease. Goals of treatment are improved mental status, metabolic parameters, and cardiopulmonary function. When the patient is stable, transition to oral levothyroxine is the goal.