02 Thyroid Disorders
Thyroid disorders are divided into three general groups:
- Hypothyroidism
- Hyperthyroidism
- Thyroiditis..
Hypothyroidism Causes
Major etiologies Include:
- Iodine deficiency
- Iodine excess
- Congenital hypothyroidism
- Amiodarone, lithium
- Thyroiditis
- Hashimoto thyroiditis (#1 cause with sufficient dietary iodine)
- Subacute thyroiditis
- Riedel's
- Surgical thyroid removal or thyroid ablation (surgical or I-131 radiation)..
Octreotide inhibits TSH secretion..
Cholestyramine inhibits thyroid hormone absorption in gut..
Iodine Excess

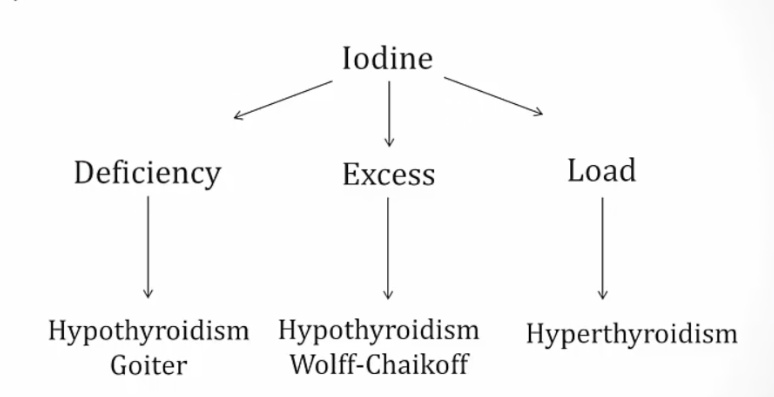
Amiodarone

Iatrogenic hypothyroidism

Child Hypothyroidism
Term used to describe arrested physical and mental development due to congenital hypothyroidism. Testing of all newborns for hypothyroidism (elevated TSH) is standard of care, because mental retardation can be minimized when thyroid hormone is administered during the neonatal period.
Most common treatable cause of mental retardation
T4/TSH newborn screening. Heel-stick blood specimens..
Babies with low thyroid hormones are asymptomatic because maternal T3/T4 cross placenta..
Most commonly caused by thyroid dysgenesis in the US. This may be secondary to maternal hypothyroidism, thyroid agenesis, iodine deficiency, or dyshormonogenic goiter.
 .
.
Symptoms:
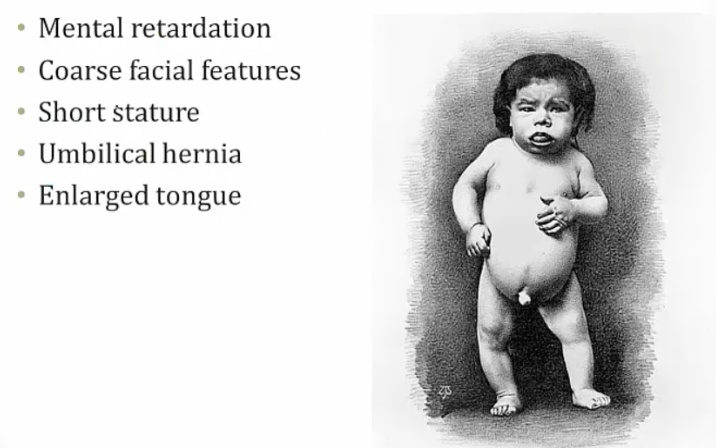
Mental retardation from lack of CNS maturation.
Coarse facial features, including wide-set eyes and a broad flat nose may be present in an infant with congenital hypothyroidism. Infants are also described as having a characteristic "puffy face".
Congenital hypothyroidism may present with a hoarse cry, weak cough, and slow respiratory effort
Prolonged neonatal (physiologic) jaundice may result from congenital hypothyroidism.
The arrest of physical development associated with congenital hypothyroidism may result in enlarged fontanelles, delayed dentition and retarded bone age.
An infant with congenital hypothyroidism may have dry skin and scarce lanugo (unpigmented downy hair), as well as a pale body with mottled cool extremities..
Hypothyroidism Symptoms
Describes a condition in which the thyroid gland does not produce enough thyroid hormone. Primary hypothyroidism accounts for >95% of cases, and describes a group of abnormalities related to the thyroid gland itself..
Results in signs and symptoms that reflect the abnormal function of many organ systems. Generally metabolism slows down:

.
CV Symptoms
Include:
- Peripheral vasoconstriction to maintain body heat, which leads to cool, pale, dry skin
- Decreased synthesis of cardiac β1 receptors, which leads to
- Bradycardia
- Decreased contractility and decreased stroke volume (decreased pulse pressure)
- Decreased cardiac output, which decreases exercise capacity and dyspnea on exertion
- Blunted EKG voltages
- Accelerated atherogenesis, due to increased total cholesterol and LDL levels (LDL receptor expression regulated by thyroid hormone).
CNS Symptoms
Include:
- Slow deep tendon reflexes, with a prolonged relaxation phase
- Fatigue and lethargy
- Mental slowness
- Perinatal mental retardation.
MSK Symptoms
Include weakness, cramps, myalgias and increased serum creatinine kinase.
Dermatologic symptoms
Include:
- Coarse, brittle hair and nails
- Yellow/orange, dry skin
- Facial and periorbital myxedema
- swelling of the skin and underlying tissue gives the skin a 'waxy' appearance.
GI Symptoms
Include decreased GI motility, which manifests as constipation.
Endocrine symptoms
Include hyperprolactinemia (if primary or secondary hypothyroidism). The decrease in T3 and T4 production increases TRH, which stimulates prolactin release.
Metabolic Symptoms
Include:
- Decreased basal metabolic rate, resulting in a decreased O2 consumption which effectively reduces respiratory rate
- Positive nitrogen balance (anabolic), which leads to weight gain
- Decreased heat production, which decreases sweating and results in cold intolerance
- Growth retardation.
Myxedema
Aka thyroid dermopathy.
Dermatologic conditions association with hypothyroidism. Symptoms include:
- Non-pitting edema of the skin
- Usually facial or periorbital swelling
- Deepening of voice (larynx swelling) and large tongue
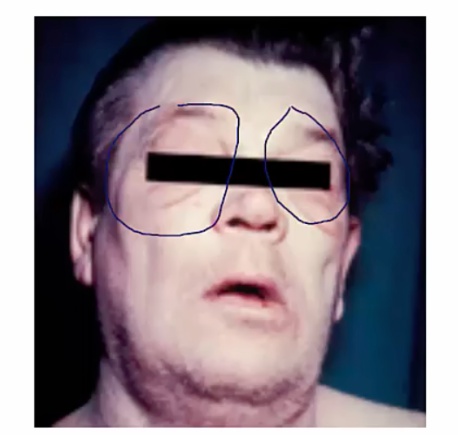
.
Hyaluronic acid is a glycosaminoglycan, a mucopolysaccharide, in the dermis. It is an osmotic agent that draws water into the interstitium, resulting in swelling. In longterm hypothyroid state, hyaluronic acid deposits in the dermis.
Pretibial myxedema
Special form of myxedema over shin. Localized dermopathy characterized by thickening of skin overlying non-pitting edema.
Occur in hyperthyroid states (notably Graves disease).
Myxedema coma
A serious complication of hypothyroidism, which generally occurs in patients with long-standing hypothyroidism that have not been properly treated. Is the only emergent hypothyroid condition.
Symptoms are coma that includes:
- Hypothermia
- Hypoventilation
- Hypotension.
Can have a spontaneous onset, or it can be precipitated by:
- Cold exposure
- Infections
- Drugs (CNS depressants)
- Hypoxia.
Treatment include:
- Respiratory support
- Intravenous levothyroxine (see below)
- Cortisol
- warmth, fluids, hypertonic saline
The use of T4 alone, combined T4 and T3, or T3 alone is controversial. Deiodinase conversion of T4 to the active hormone T3 is often reduced in these patients, advising T3 administration. However, T3's more immediate action and short half-life may be more likely to cause arrhythmias (particularly with compromised myocardial function).
Hyponatremia

Hypothyroidism Treatment
Usually just gives T4..

Hyperthyroidism Causes
Aka thyrotoxicosis.
The most common cause is Graves disease. Other causes:
- Toxic multinodular goiter
- amiodarone
- iodine load
- early thyroiditis.
Graves
Females are affected 10x more frequently than males, with a peak incidence between age 20-40.
Is caused by a type II hypersensitivity reaction.
A decrease in self-tolerance to thyroid auto-antigens leads to the production of autoantibodies.
Thyroid-stimulating immunoglobulin (TSI) is an IgG that binds and activates TSH receptor, mimicking the action of TSH. TSI is relatively specific the disease.
TSI, Anti-thyroglobulin, and anti-thyroid peroxidase antibodies are often present.
Hyperthyroidism results from continuous stimulation of the thyroid gland by IgG autoantibodies. This stimulation leads to diffuse, symmetrical, nontender and hyperfunctional enlargement of the thyroid (aka diffuse goiter)..
Associated with HLA-DR3 and HLA-B8.
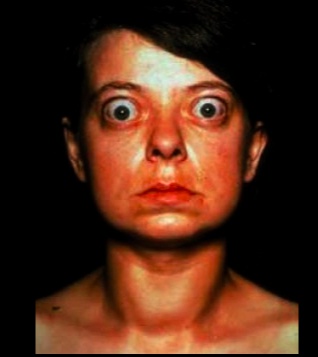
Eye symptoms is characterized by:
- exophthalmos (bulging eyes)
- proptosis (protrusion of eye)
- periorbital edema
- impairment of the extraocular muscles
- No ocular symptoms
 .
.
Pretibial myxedema
Infiltrative dermopathy. A scaly thickening and induration of the skin overlying the shins with nonpitting edema, results from TSH receptor stimulation on dermal fibroblasts. This only occurs in 1-2% of patients.
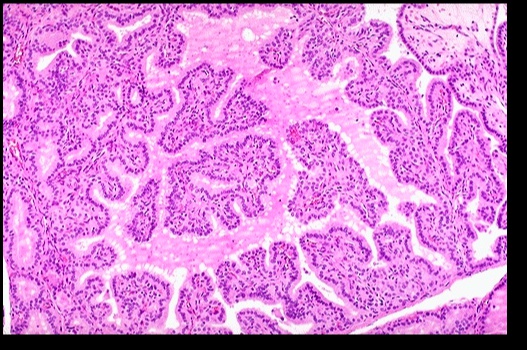
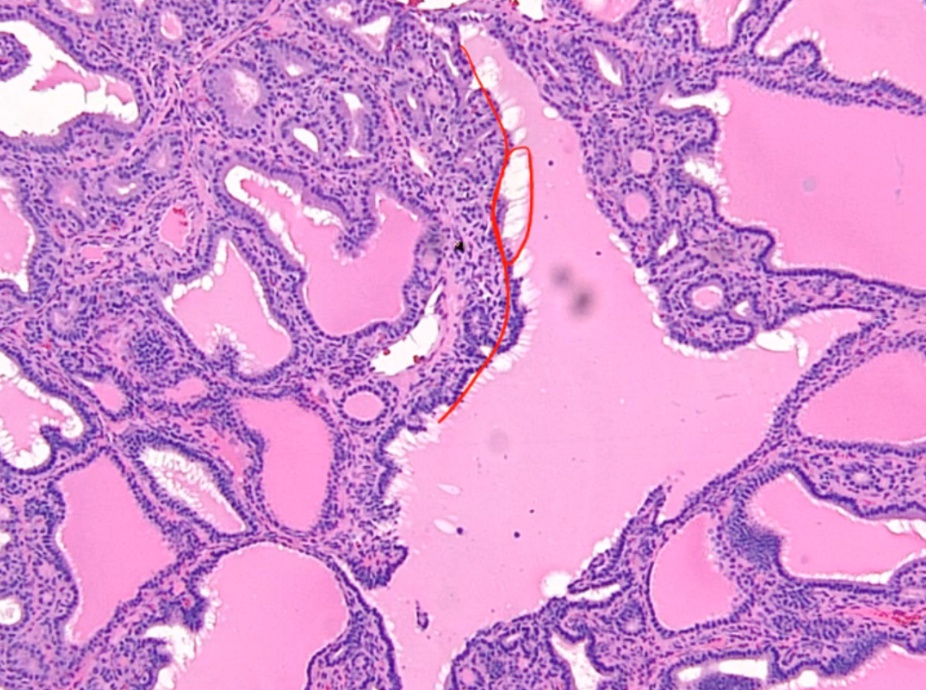
Histologic findings include:
- Diffuse hypertrophy and hyperplasia of thyroid follicular epithelial cells
- Colloid appears pale with scalloped (moth-eaten) margins due to increased colloid absorption.
- Lymphocytic infiltrate leads to germinal centers in the thyroid (more commonly seen in Hashimoto thyroiditis). **
.
The most common treatment in the U.S. is radioactive iodine (131I)..

Graves ophthalmopathy follows its own course..

Steroids for inflammation because of lymphocytes.
Thyroid Storm
A life threatening complication of Graves disease. It describes an abrupt-onset of severe thyrotoxicosis.
Is most likely induced by an acute massive elevation in catecholamine levels in patients with preexistng hyperthyroid disease, as seen in situations of acute stress including infection, surgery, trauma.
Most commonly seen in patients with Graves disease, as well as other hyperthyroid disorders such as toxic multinodular goiter.
Patients will present with:
- High fever
- Delirium
- Tachycardia with death from arrhythmia (out of proportion to fever)
- Diarrhea
- Vomiting
- Coma.
Typical lab results include:
- Decreased TSH
- Increased T3 and T4
- Elevated liver enzymes
- Hyperglycemia from catecholamines and thyroid hormone
- Hypercalcemia from bone turnover.
Treated with the 4P's and bile acid resins:
- Propranolol (propranolol or esmolol)
- Propylthiouracil (PTU)
- Prednisone (and other corticosteroids)
- Potassium iodide compounds (e.g. Lugol iodine)
- Bile acid resins (e.g. cholestyramine)
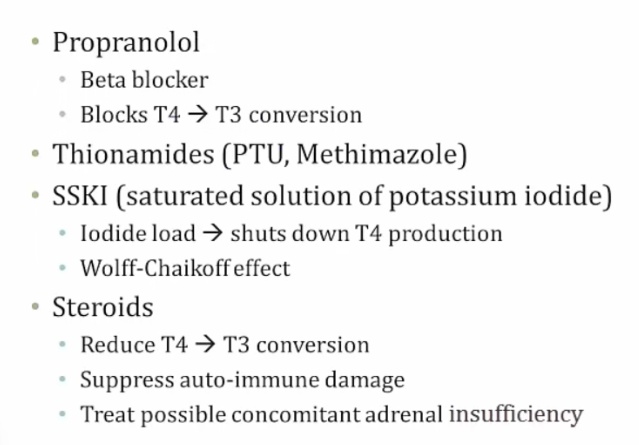
Adrenal insufficiency from thyroid storm depleting cortisone.
Complications include:
- Tachyarrhythmias (notably atrial fibrillation and ventricular fibrillation) which are the most common cause of death in patients with thyroid storm
- Shock (heart failure, diarrhea/vomiting-induced volume depletion)
- Coma.
Goitrogens
Substance that disrupts the production of thyroid hormones by interfering with iodine uptake in the thyroid gland. This triggers the pituitary to release TSH, which then promotes the growth of thyroid tissue, eventually leading to goiter.
Examples include:
- Iodine, most common
- Lithium, used in psychiatry, inhibit thyroid hormone release
- Certain foods (cassava, plant; millet, grass)
- Many cruciferous vegetables are goitrogens (broccoli, brussel sprouts).
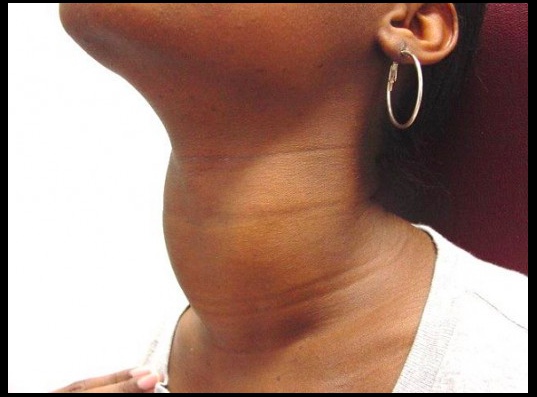
Goiter
An enlarged thyroid gland, which has undergone hyperplasia in response to excessive stimulation of TSH receptors.
There are three types of goiters:
- Simple
- Multinodular
- Dyshormonogenetic..
A fixed, painless and hard (rock-like) goiter.
Autoantibodies stimulate TSH receptors, resulting in the formation of a diffuse goiter.
Simple goiter
Occurs when the body is unable to produce sufficient thyroid hormone, and the thyroid gland undergoes hyperplasia in response. In many cases the etiology is unknown, but it may be caused by a lack of dietary iodine or intrinsic thyroid hormone production defects.
Simple goiter:
Patients with iodine deficiency goiters are most commonly euthyroid. Goitrous hypo- and hyperthyroidism may also be seen, but this is less common. See endemic goiter..
Multinodular goiter
Describes an enlarged thyroid gland with multiple nodules, typically due to relative iodine deficiency. Patients are usually euthyroid.
Multilodular goiter
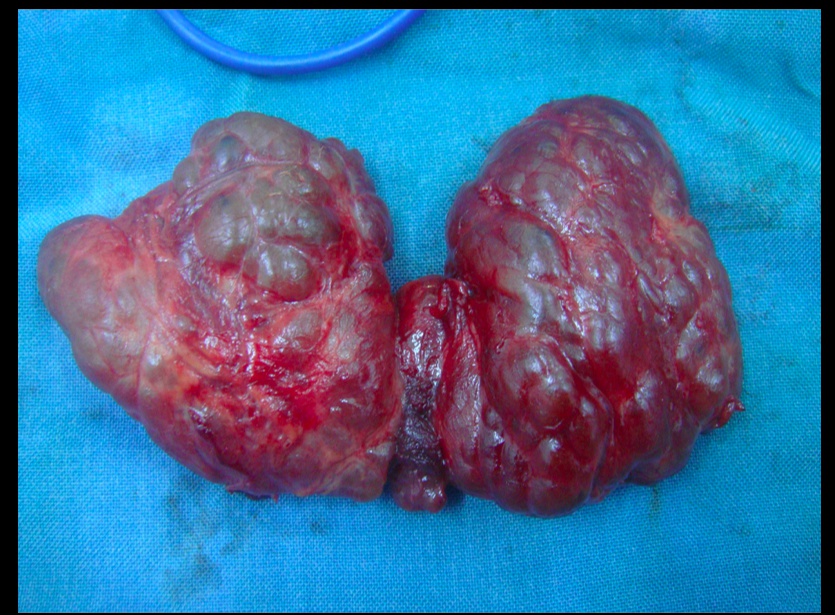
Toxic multinodular goiter
Toxic adenomas: 1 nodule Toxic multinodular goiter: multiple nodules..
Contains focal patches of hyperfunctioning follicular cells that function independently of thyroid stimulating hormone (TSH) due to a mutation of the TSH receptor. The term 'toxic' does not mean malignancy, rather it refers to the hyperthyroidism (thyrotoxicosis) that results from these self-sufficient nodules.
As a result of the autonomously functioning thyroid nodules, the release of T3 and T4 is increased resulting in hyperthyroidism.
Treatment is surgery or radioactive iodine to differentiate between benign nodules and cancer..
Endemic goiter and iodine deficiency
Simple/multinodular goiter caused by dietary iodine deficiency; The most common cause of hypothyroidism worldwide.
Commonly found in mountainous areas (iodine depleted by run-off).
Constant elevation of TSH result in enlarged thyroid..

Dyshormonogenetic
Genetically-determined thyroid hyperplasia that result from enzyme defects in thyroid hormone synthesis, most commonly thyroid peroxidase. These deficiencies are a cause of congenital hypothyroidism (cretinism).
Jod-Basedow
Describes iodine-induced hyperthyroidism. It arises in two settings:
- Administration of iodine to a patient with an iodine deficiency goiter (e.g. patient with endemic goiter relocates to iodine-abundant geographical location)
- Patients with toxic adnomas, hot nodules, or Graves disease:
- Drugs with high iodine content
- Exectorants (potassium iodide)
- CT contrast dyes
- Amiodarone.
Hyperthyroidism symptoms
Results in signs and symptoms that reflect the abnormal function of many organ systems. These include:

.
CV Symptoms
Include:
- Peripheral vasodilation to get rid of heat which leads to warm, flushed skin
- Increased synthesis of cardiac β1 receptors which leads to:
- Increased heart rate (sinus tachycardia)
- Increased contractility and increased stroke volume (increased pulse pressure)
- Increased cardiac output
- Cardiomegaly, systolic hypertension, high-output heart failure
- Hypercalcemia-induced EKG changes (due to increased bone turnover),
- Arrhythmias (especially atrial fibrillation) secondary to increased heart rate and/or cardiomegaly.
CNS Symptoms
Include an increased sympathetic nervous system tone. This leads to:
- Fine tremor (hands)
- Anxiety
- Insomnia
- Emotional lability
- Inability to concentrate
- Fast deep tendon reflexes.
Eye Symptoms
Are secondary to the increase of sympathetic nervous system tone. Overstimulation of the superior tarsal muscle (elevate the upper eyelid) leads to a wide-eyed staring gaze and lid lag
TSI & other autoantibodies cross-react with and stimulate several tissues in the body containing TSH receptors, including:
- Thyroid
- Retro-orbital fibroblasts (activated by T lymphocyte)
- Dermal fibroblasts (activated by T lymphocyte)
When stimulated, TSH receptors on orbital pre-adipocyte fibroblasts synthesize hydrophilic glycosaminoglycans (notably hyaluronic acid and chondroitin sulfate). The accumulation of glycosaminoglycans, infiltration of fat and T cells in the retro-orbital space, and edema of the extraocular muscles all lead to exophthalmos (proptosis). This occurs in 50% of patients..
NOTE that true thyroid ophthalmopathy with exophthalmos (proptosis) is only seen in Graves disease..
GI Symptoms
Increasing GI motility, which leads to malabsorption and diarrhea.
Endocrine Symptoms
Include:
- Increased bone turnover, leading to osteoporosis and an increased risk of fractures
- Oligomenorrhea.
Metabolic Symptoms
Include:
- Increased basal metabolic rate, resulting in increased O2 consumption which effectively
- increases respiratory rate (tachypnea)
- Weight loss, despite an increased appetite
- Negative nitrogen balance (catabolic), which can lead to thyroid myopathy (proximal muscle weakness and decreased muscle mass)
- Increased heat production and sweating, leading to heat intolerance.
Hyperthyroidism treatments
Treatment includes:
- β-blockers (propanolol)
- Thioamides
- High-dose iodide
- Radioactive iodine (131I).
B-Blockers
Are indicated to control the symptoms of increased sympathetic nervous system tone. Furthermore, propranolol decreases peripheral conversion of T4 to T3 by interfering with the activity of 5'-deiodinase, producing a net decrease in thyroid hormone actions at target tissues.
Thioamides
Include PTU and methimazole.
Thioamides may induce remission by blocking new thyroid hormone production via inhibition of the organification and coupling steps of thyroid hormone synthesis. In addition, PTU (but not methimazole) also inhibits peripheral conversion of T4 to T3..
SE include:

.
Methimazole
A teratogen.
Use PTU in pregnancy, especially 1st trimester. Methimazole better for long term because less hepatotoxicity also good in breastfeeding (not found in breast milk)..
High-dose iodide
Blocks the release of T4 and T3 into circulation and transiently inhibits thyroid hormone synthesis by blocking the organification step (Wolff-Chaikoff effect).
Iodine 131I
Different from the 123I used in imaging studies. Becomes concentrated in the thyroid gland. This leads to ablation of follicular cells, and decreases thyroid function..
Thyroiditis
Thyroiditis can cause either hypothyroidism or hyperthyroidism:
- Early state: Inflammation causes more secretion of thyroid hormones and thus hyperthyroidism
- Late state: Eventually, thyroid is burned out and produces less thyroid hormones, thus hypothyroidism..
Hashimoto thyroiditis
The most common cause of hypothyroidism in iodine-sufficient regions, or developed countries.
Aka chronic autoimmune thyroiditis.
Histologically, defined by:
- Lymphocytic infiltrate (germinal centers)
- Atrophic thyroid follices lined by Hürthle cells (pink epithelial cells).
Chronic inflammation, germinal centers and Hurthle cells:
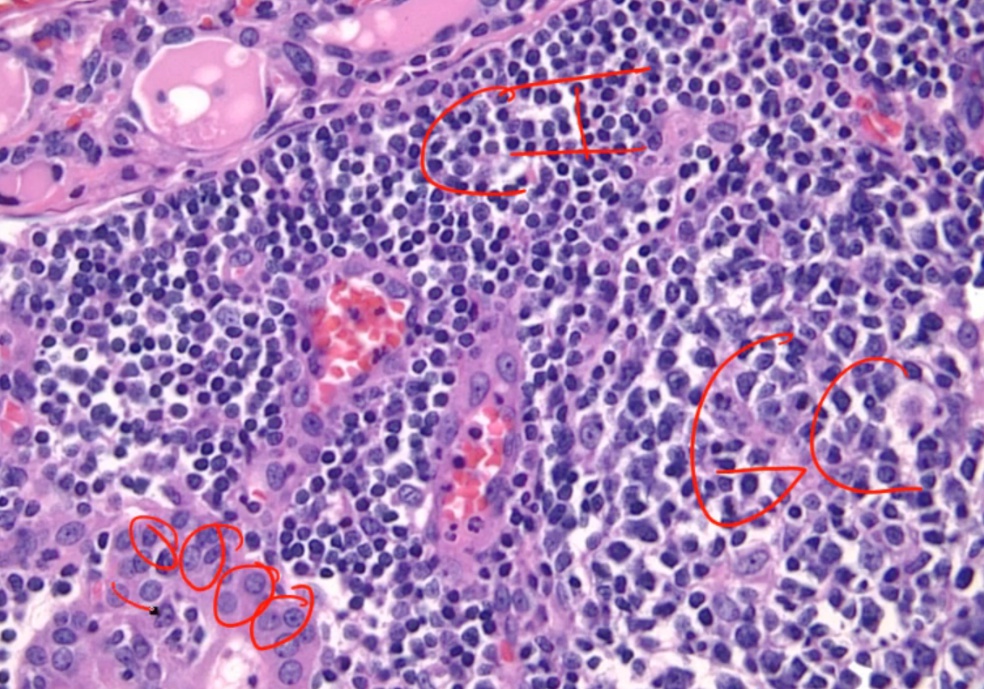
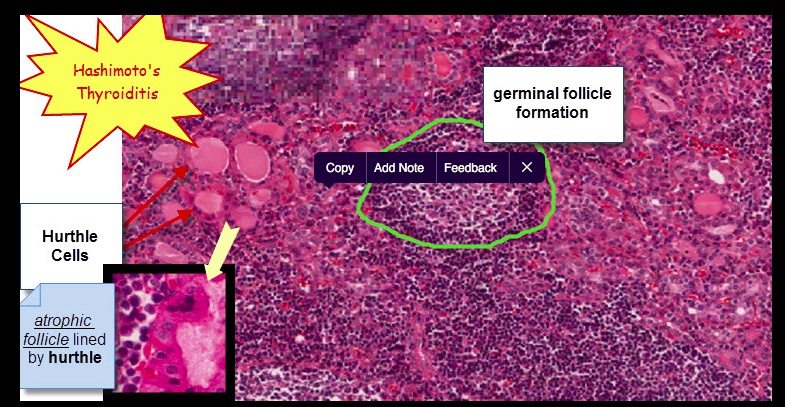
Because thyroid follicles are normally lined by low cuboidal epithelium, this is also known as Hürthle cell metaplasia.
Caused by autoimmune-mediated destruction of the thyroid gland. Thyroid cell death results from a failure of self-tolerance to thyroid auto-antigens:
- CD8+ T-cell mediated cell death
- Activated TH1 cells release IFN-γ that recruits and activates macrophages, which release inflammatory mediators that inflict follicle damage.
- Anti-thyroid autoantibodies (anti-Tg and anti-TPO) from B cells lead to complement activation and antibody-dependent cell-mediated cytotoxicity.
Include anti-thyroglobulin antibodies and anti-thyroid peroxidase (TPO) antibodies (markers of thyroid damage). But no TSI. Note that TPO was formerly known as microsomal antigen.
Is associated with HLA DR3 and DR5.
Presents as a nontender thyroid, which often becomes diffusely enlarged. The development of hypothyroidism (and resultant symptoms) is insidious, most commonly in women age 30-50.
Early in the course of Hashimoto thyroidis, hyperthyroidism may occur as thyroid follicles rupture, causing thyrotoxicosis.
Increased risk of developing: Other autoimmune diseases, for example:
- Primary thyroid lymphoma, particularly B-cell non-Hodgkin lymphomas (extranodal marginal zone lymphomas of mucosa-associated lymphoid tissue [MALT] type).
- Type 1 Diabetes Mellitus
- Systemic Lupus Erythematosus
- Sjögren’s syndrome
- Myasthenia Gravis
- Pernicious anemia.
The treatment for hypothyroidism associated with Hashimoto thyroiditis is levothyroxine..
Acute suppurative thyroiditis
Often occurs secondary to bacterial infection, such as with Staphylococcus aureus.
Symptoms include:
- Fever
- Painful thyroid
- Painful cervical lymphadenopathy
- Gland destruction leads to an initial thyrotoxicosis, with labs including:
- Increased serum T4
- Decreased serum TSH
- Decreased I-123 uptake.
Because of a frequent bacterial etiology, this condition is commonly treated with antibiotics.
Subacute granulomatous thyroiditis
aka DeQuervain thyroiditis.
Occus in young female.
Can be caused by mumps or coxsackievirus.
Presents as a tender thyroid and is the most common cause of thyroid pain. This is in contrast to both Hashimoto thyroiditis and Riedel thyroiditis, which present without thyroid pain.
Early phase can manifest as hyperthyroidism, as the damaged gland spills T4. Over time, it can progress to a state of hypothyroidism
No lymphadenopathy, which is a different physical exam finding than with acute thyroiditis
Preceded by a flu-like illness including:
- Sore throat and fever
- Jaw pain
- Tender thyroid
- Markedly elevated erythrocyte sedimentation rate.
On histology, a lymphocytic infiltrate with multinucleated giant cells surround fragments of colloid.
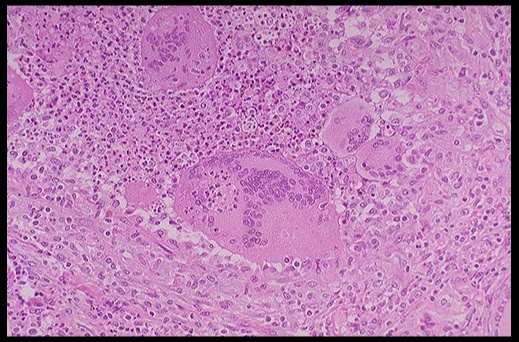
.Subacute granulomatous thyroiditis. Note the giant cells, inflammatory cells and destruction of the thyroid follicules.
The treatment is aspirin. In severe disease, cortisol may supplement this treatment. Thyroid symptoms is mild and resolve in a few weeks.
Subacute lymphocytic (painless) thyroiditis
Aka painless thyroiditis.
Variant of Hashimoto's where lymphocytes infiltrate the thyroid gland.
Presents as a nontender thyroid and transient hyperthyroidism followed sometimes by hypothyroidism:
- Transient hyperthyroidism: can look like Grave's without eye/skin findings (serum TSI not elevated)
- Hypothyroidism: can look like Hashimoto's.
Occuring most commonly in postpartum women.
On histology, appears with lymphocytic infiltrate and prominent germinal follicles. Hürthle cell metaplasia is not prominent (versus Hashimoto thyroiditis, in which Hürthle cell metaplasia is a prominent feature).
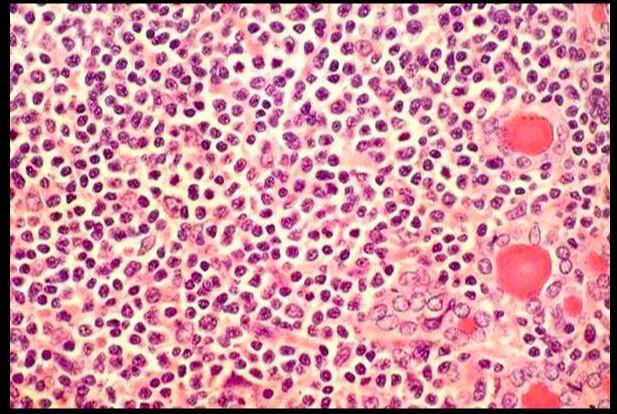 .Lymphcytic inflitrate seen in subacute lymphocytic thyroiditis.
.Lymphcytic inflitrate seen in subacute lymphocytic thyroiditis.
Usually self limited (weeks). Roughly one third of patients develop hypothyroidism within 10 years.
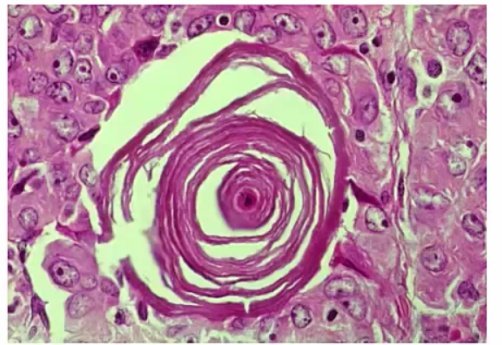
Riedel Thyoiditis
Occurs when fibrous tissue (collagen) replaces thyroid parenchyma, causing the patient to be hypothyroid.
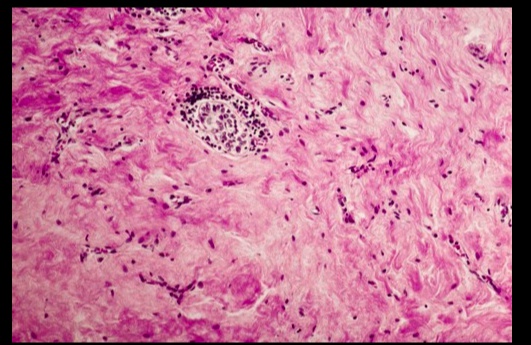 .Riedel Thyroiditis. Note how the thyroid gland parenchyma has been overrun by fibrous tissue.
.Riedel Thyroiditis. Note how the thyroid gland parenchyma has been overrun by fibrous tissue.
The thyroid gland is:
- Fixed
- 'Hard as wood' or 'rock-like'
- Painless
Often extends beyond the thyroid:
- parathyroid gland: hypoparathyroidism
- recurrent laryngeal nerves: hoarseness
- trachea compression: difficulty breathing.
Classified as an IgG4-related systemic disease, which is characterized by:
- Lymphoplasmacytic tissue infiltrates with abundant IgG4-producing plasma cells
- Storiform fibrosis
- Elevated serum IgG4 concentrations.
Fibrosis extends beyond the thyroid capsule and into the surrounding tissue in both Riedel thyroiditis and anaplastic carcinoma. A patient with Riedel thyroiditis will be younger (average age 40) than a patient with anaplastic carcinoma, and malignant cells will be absent on biopsy..
IgG4 related diseases
Include:
- Autoimmune pancreatitis
- Non-infectious aortitis
- Retroperitoneal & mediastinal fibrosis
- Riedel thyroiditis.
Thyroid Cancer
- Thyroid is bloody organ. Do not cut open

Types:
- Papillary carcinoma: most common
- Follicular carcinoma
- Medullary Carcinoma
- Anaplastic Carcinoma.
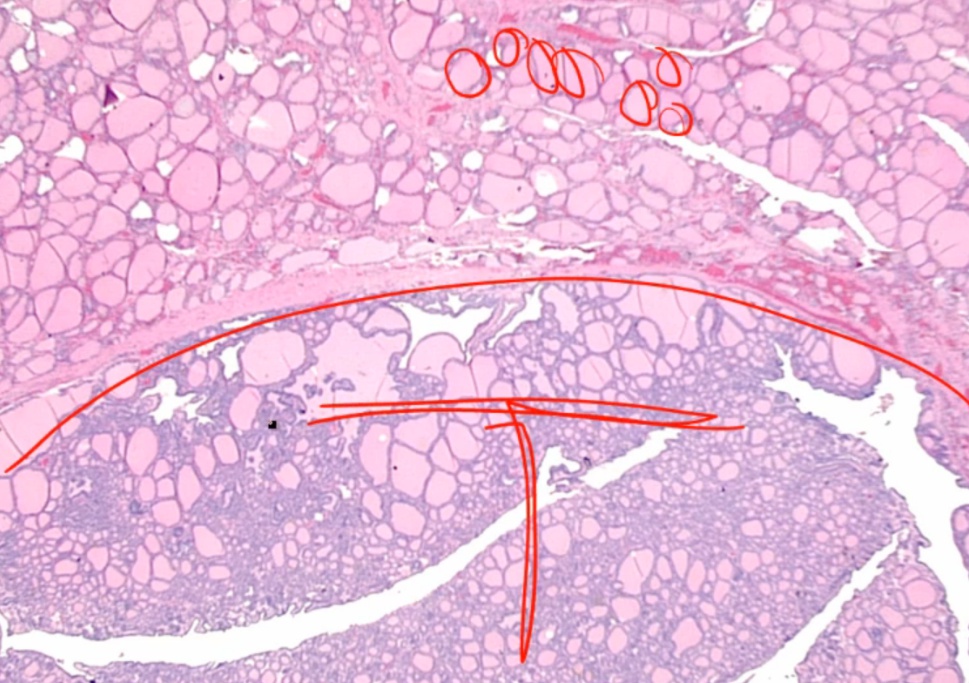
Follicular Adenoma

.
Looks like follicles in tumor with capsule around it.
Follicular Carcinoma

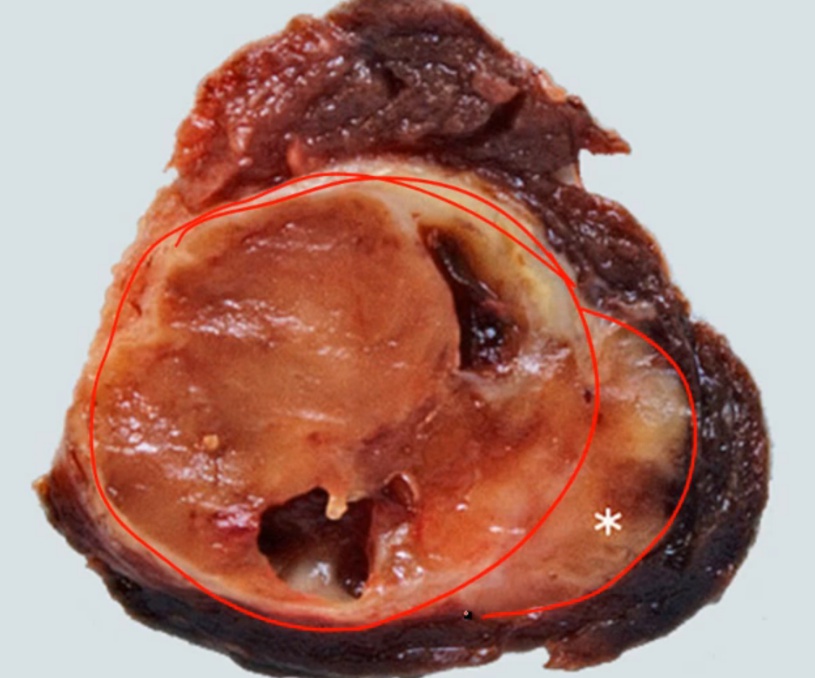
.
Spread hematogenously.
Cancers spreading hematogenously
Cancers:
- RCC
- Hepatocellular carcinoma
- Follicular carcinoma
- Choriocarcinoma.
FNA only examines cells and not capsules, thus cannot make distinction between the two.
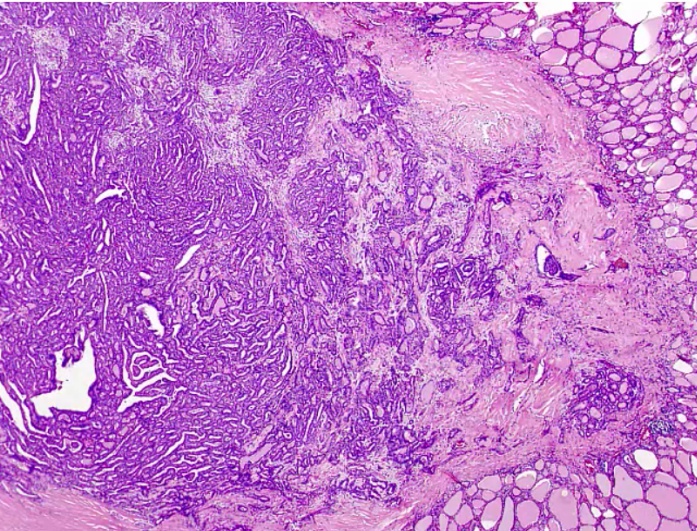
Papillary Carcinoma
- association with protooncogene BRAF, serine/threonine kinase.
Cause:
- acne radiation
 .
.
Histology:
- Orphan Annie eye nuclei: no nuclei
- Nuclear grooves: line through nucleus
- Psamomma bodies
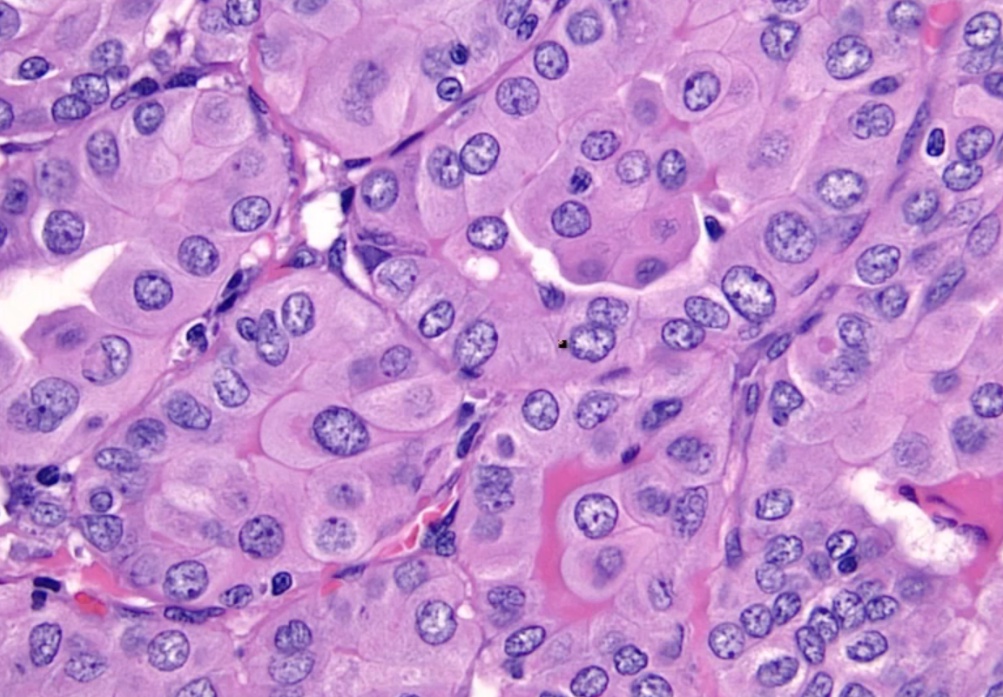
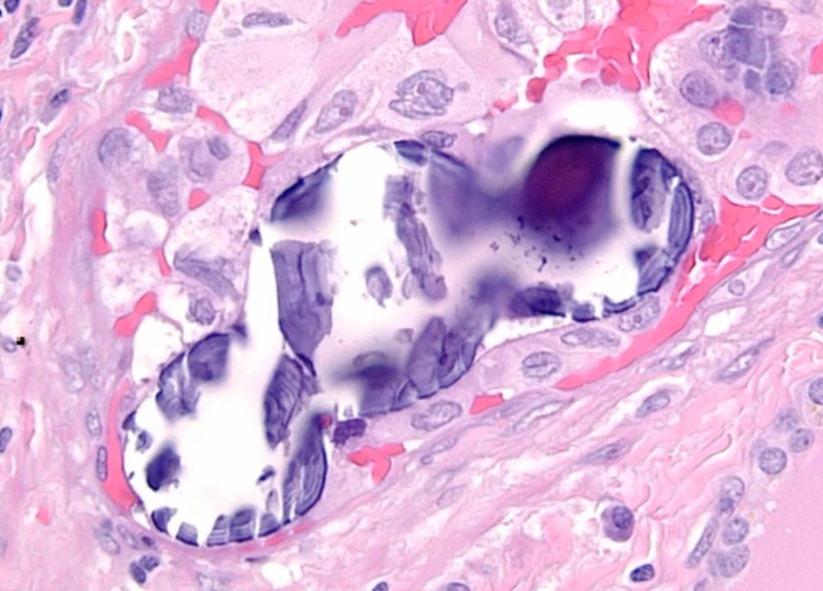
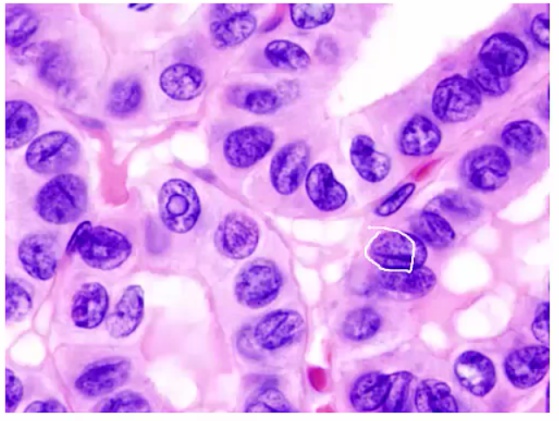
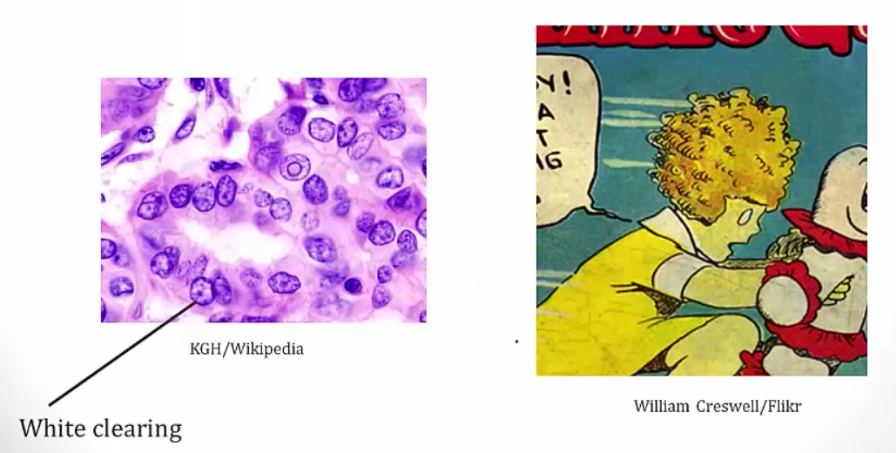
.
Spread to cervical but excellent prognosis.
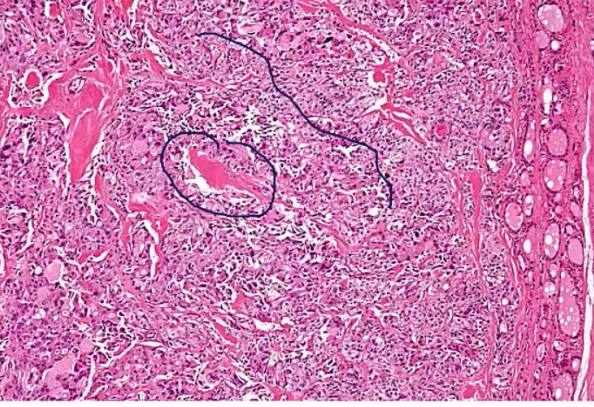
Medullary Carcinoma

- flushing and diarrhea.
Histology:
- malignant cells in amyloid stroma
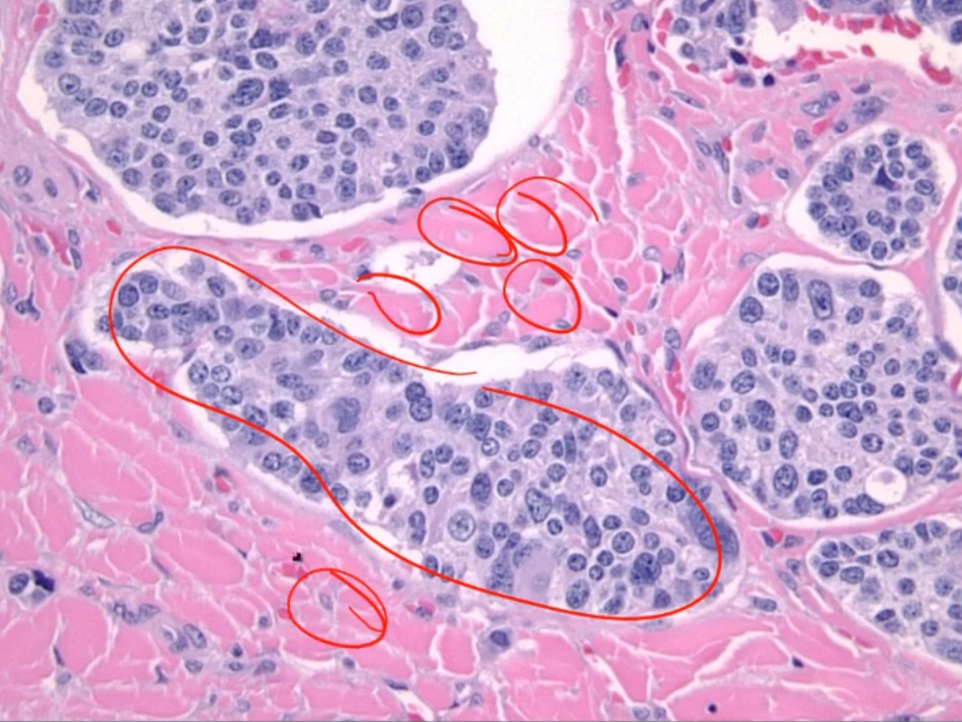
 .
.
Association:
 .
.
Treated with surgery because does not take up iodine.
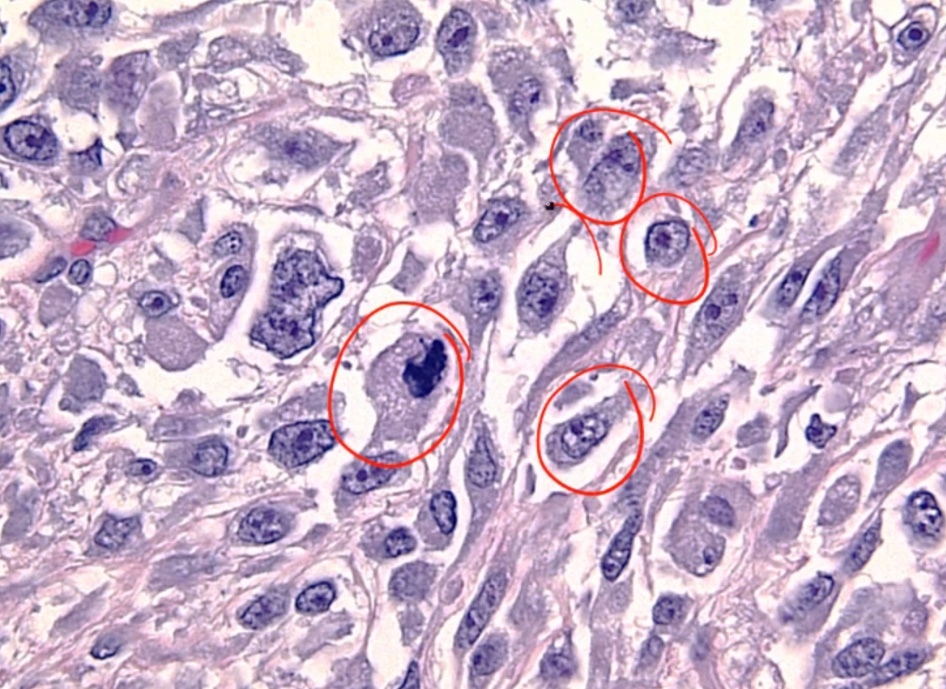
Anaplastic Carcinoma
 .
.
 .
.
Similar to Riedel
 .
.
Histology shows undifferentiated cells that look like nothing else
 .
.
Misc
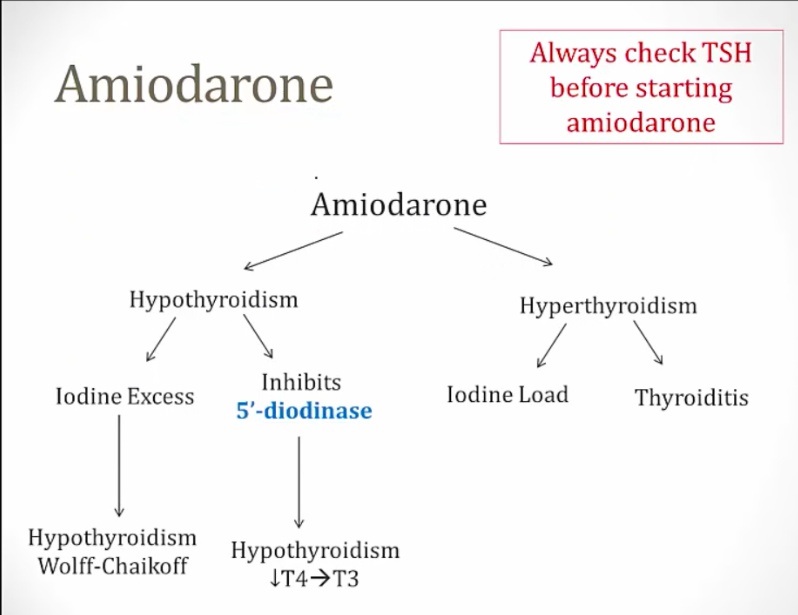
Amiodarone
Can cause both hypo and hyperthyroidism:
- Iodine excess and inhibition of 5-deiodinase causes hypothyroidism
- Can cause hyperthyroidism in patients with thyroid disease and destructive thyroiditis in patients with no thyroid disease..

Iodine
can cause both hypo and hyperthyroidism:
- Chronic deficiency causes hypothyroidism and goiter because of constant TSH elevation stimulating thyroid.
- Chronic excess causes hypothyroidism via Wolff-Chaikoff effect.
- In patients with toxic adenomas and endemic goiters, a load can cause hyperthyroidism via Jod-Basedow effect..

Oligomenorrhea
In hypOthyroidism-- you have decreased t3 and decreased t4. This causes TRH to be INCREASED ( trh from hypothalmus)--> We know that TRH stimulates PROLACTIN and hyperprolactinemia cuases decreased LH/FSH --> amenorrhea..
HypERthyroidism can also cause amenorrhea. The mechanism behind this involves SHBG regulation ( produced in liver). So, hyperthyroidism leads to Increased production of SHBG-- this leads to decreased amount of free estrogen --> decreased chances that you will have an LH peak and therefore AMENORRHEA..
Labs
- Primary thyroid dysfunction are caused by thyroid dysfunction (most common)
- Secondary thyroid dysfunction are caused by pituitary dysfunction
- Tertiary are caused by hypothalamic dysfunction..
Primary hypo/hyperthyroidism labs
TSH is opposite thyroid hormone:
- Hypothyroidism = high TSH and low T3/T4
- Hyperthyroidism = low TSH and high T3/T4.
Central hypo/hyperthyroidism labs
Aka secondary or tertiary thyroid dysfunction.
TSH parallels thyroid hormones:
- Hypothyroidism = low TSH and low T3/T4
- Hyperthyroidism = high TSH and high T3/T4.
Causes:

In the rare case that a patient has secondary hyperthyroidism due to a TSH-secreting pituitary adenoma, inject TRH and look for an increase in TSH..
Best initial test because it tests at tissue level, and doesn't flucuate like T3/T4, which test at serum level. TSH is most sensitive test..
Tertiary hypothyroidism
TSH levels will be normal/low-normal, but in rare cases may be elevated. For this reason, TSH and free thyroxine are examined concurrently when central hypothyroidism is expected..
Reverse T3
Usually parallels T4 levels..
Used in Euthyroid sick syndrome.
- Critically ill patient will have low TSH and low T3/T4 that looks like central hypothyroidism
- rT3 rises in critical illness due to impaired clearance
Checking rT3 in critically ill patient will tell whether patient has central hypothyroidism or sick euthyroid syndrome.
T3 Toxicosis
An increase in serum T3 with low or normal serum T4. Can be seen in any condition that causes hyperthyroidism, and the treatment is the same as for hyperthyroidism.
Other lab findings include:
- increased bone turnover, and therefore an increase in serum calcium (hypercalcemia),
- increased glycogenolysis, and therefore an increase in serum glucose (hyperglycemia), and
- increased low densitiy lipoprotein (LDL) receptor synthesis, and therefore a decrease in serum cholesterol (hypocholesterolemia)..
123I
- Increased diffused uptake: Graves
- Decreased diffused uptake: Hashimoto, thyroiditis, exogenous thyroid
- Hot nodules: Toxic multinodular goiter
- Cold nodules: Adenoma or cancer, thus FNA needed
patches of multinodular goiter

Excess thyroid hormone negatively feeds back on the pituitary, leading to decreased TSH. This decrease in thyroid activity manifests as decreased 123I uptake, with thyroid gland atrophy..