menopause
-
related: OBGYN
Hot flash
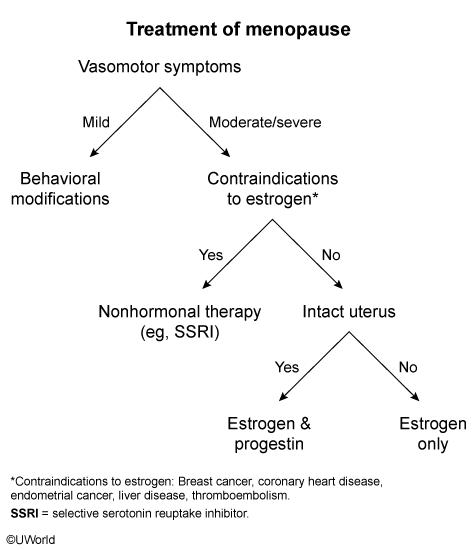
- first line: estrogen based hormone replacement therapy
- add progestone for people with intact uterus
- CI include history of thromboembolism, coronary heart disease, breast cancer, and endometrial cancer.
- nonhormonal treatment: selective serotonin reuptake inhibitor (eg, fluoxetine) or serotonin-norepinephrine reuptake inhibitor (eg, venlafaxine).
- 2nd line: clonidine is a second-line option for women who cannot take estrogen and do not have adequate relief from selective serotonin reuptake inhibitors. However, it provides only a modest benefit and is associated with significant side effects (eg, dry mouth, constipation, dizziness).
Combination estrogen/progesterone menopausal hormone therapy (MHT), or estrogen alone for women who have had a hysterectomy, is the most effective treatment available for menopausal hot flashes. Combination MHT is associated with an increased risk of stroke, coronary heart disease (CHD), breast cancer, and venous thromboembolism. However, much of this risk is age-related; women age 50-59 have a lower risk of adverse effects, resulting in a more favorable risk-benefit profile compared with women age >60. In light of this, MHT can be safely used for a short period (3-5 years) in younger, low-risk women.
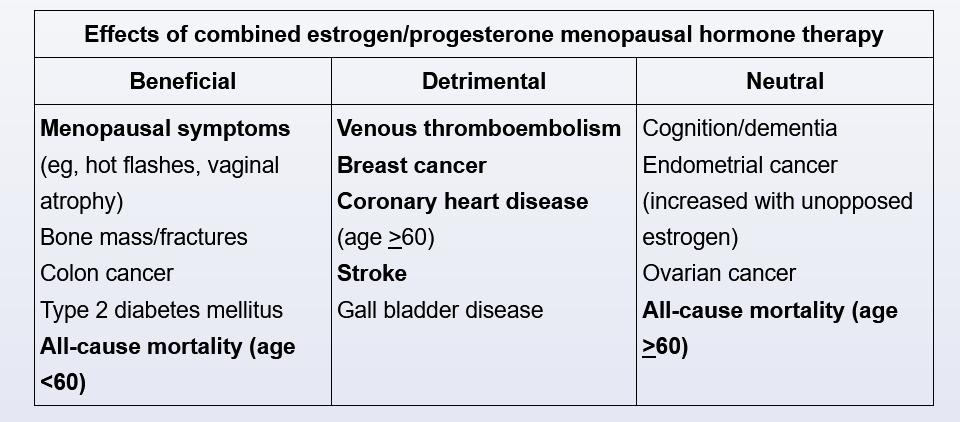
Major contraindications to MHT (even in younger women) include established CHD, active liver disease, and a history of breast cancer, venous thromboembolism, or stroke. The risk can be further stratified using a standardized cardiovascular risk calculator (eg, American Heart Association/American College of Cardiology calculator); MHT should be avoided in patients whose estimated 10-year risk of coronary events is >10%.
SERMs
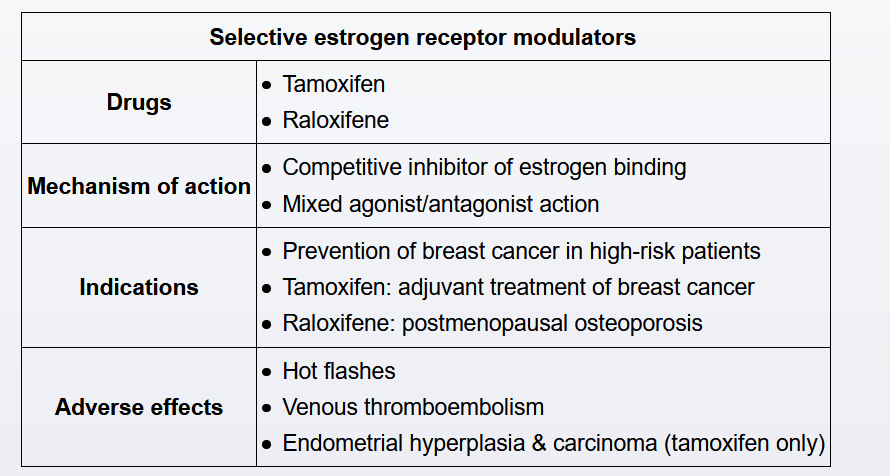
Tamoxifen is a selective estrogen receptor modulator that is used as an adjuvant therapy in patients with nonmetastatic, estrogen receptor-positive breast cancer and for breast cancer prevention in high-risk women (eg, atypical hyperplasia, age >35 with history of lobular carcinoma in situ).
Tamoxifen acts as an estrogen receptor agonist in the endometrium and is associated with an increased risk of endometrial hyperplasia/cancer and uterine sarcoma in postmenopausal women. In premenopausal women, tamoxifen is associated with endometrial polyp development, a typically benign cause of abnormal uterine bleeding. In asymptomatic women taking tamoxifen (such as this patient), regular screening has no proven benefit in detecting endometrial cancer or uterine sarcoma. Therefore, patients on tamoxifen require evaluation only if symptoms develop (eg, abnormal uterine bleeding, postmenopausal bleeding).
Tamoxifen is associated with a 2- to 3-fold increased rate of venous thromboembolic events, particularly in the first 2 years of use. Therefore, women on tamoxifen are advised to discontinue it several days prior to activities that further increase the risk of venous thromboembolism (eg, surgery, travel).
Tamoxifen does not adversely affect the risk for coronary artery disease in women with or without pre-existing disease.
In premenopausal women, tamoxifen decreases bone mineral density (likely due to its partial antagonist activity). In contrast, in postmenopausal women, tamoxifen increases bone density (likely due to its partial agonist activity) and decreases the risk of osteoporotic fracture. However, tamoxifen is not recommended for osteoporosis prevention due to its association with endometrial cancer.