ACS
Acute Coronary Syndromes
- acute or subacute disruption in blood flow
- plaque rupture unlike narrowing in stable angina
- no clear precipitant
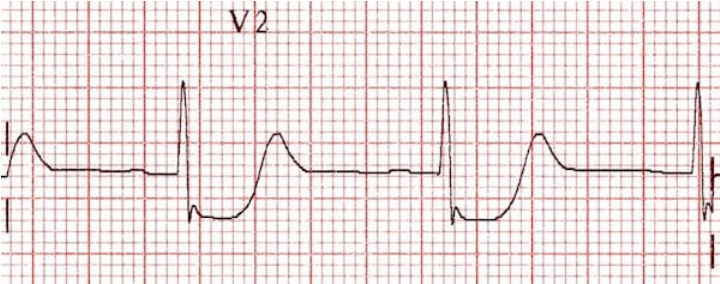
- STEMI: ST elevation of 1 mm or more in 2 or more contiguous limb or chest leads, except V2 and V3
- ST elevation in 2mm or more in men and 1.5mm or more in women in V2 and V3
- Posterior MI: 2mm ST depression in anterior leads V1-V4 with ST elevation in inferior or lateral leads
- New LBBB considered STEMI and can reflect LAD occlusion
- NSTE-ACS: divided to NSTEMI vs unstable angina based on biomarkers
- NSTEMI: positive troponin, no ST elevation
- Unstable angina: new, worsening angina, with or without ECD changes, no detectable cardiac injury markers
- High sensitivity troponin: increased diagnosis of NSTEMI
- more likely if > 13,000 pg/mL
- Biotin can increase troponin levels
STEMI
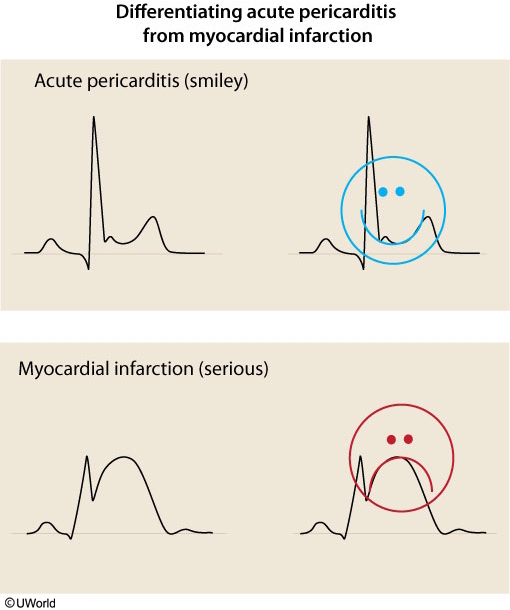
- pathogenesis: plaque rupture, platelet adhesion/activation/aggregation, thrombosis, vessel occlusion
- transmural infarct
- ddx
- acute pericarditis
- myopericarditis from viral illness or autoimmune condition
- LVH induced ECG changes, but usually concave in appearance
- aortic dissection: ST elevation if involve LCA or RCA. ST elevation from transmural infarct
- severe hypercalcemia: also has flat T and short QT
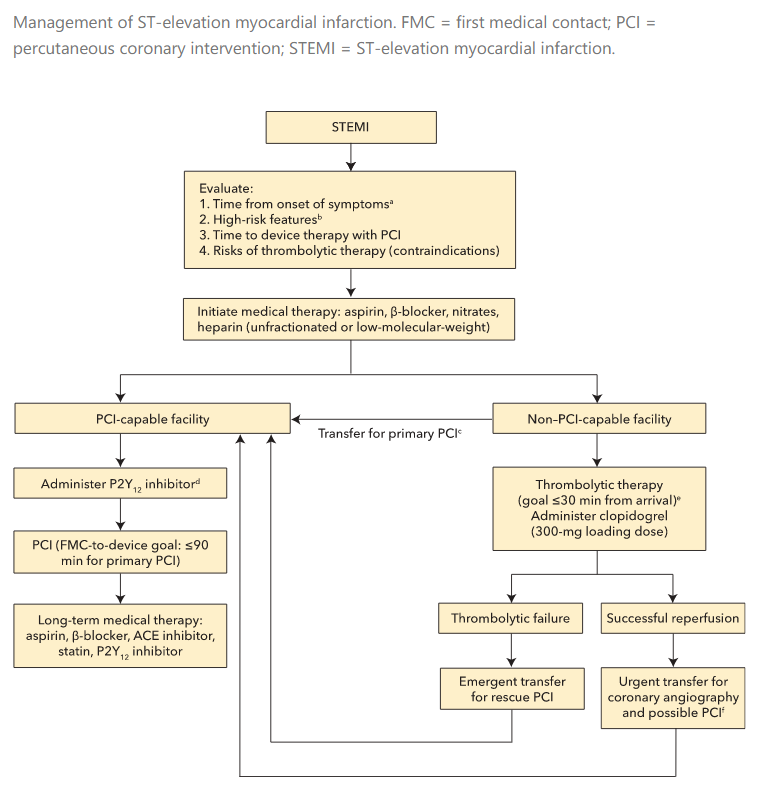
Management
- loading dose ASA
- IV heparin, lovenox, or fondaparinux
- Clopidogrel loading shown to increase rates of vessel patency
- thrombolytic or PCI. PCI preferred
- thrombolytics:
- for patients with STEMI when sx < 12 hours
- PCI not available in 120 minutes
- monitor ECG in first 1-1.5 hours after thrombolytic to confirm at least 50% improvement in ST elevation
- transfer to PCI center if not achieved reperfusion
- PCI
- Ideal time: 90 min from first contact, 120
- get ASA and heparin before therapy
- during therapy, patients will receive heparin with or without glycoprotein 2b/3a blockade or bivalirudin, stents with P2Y12 inhibitor
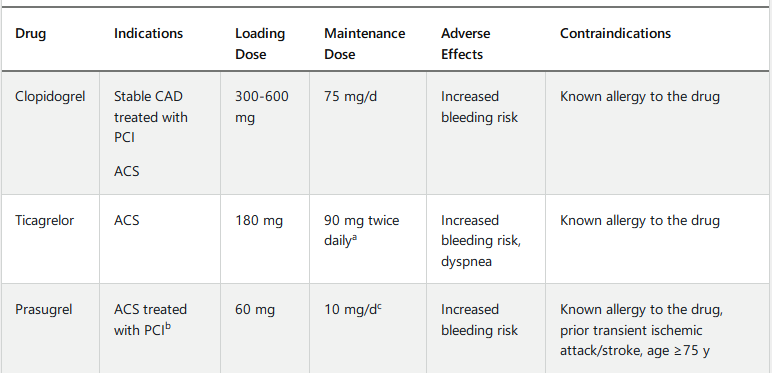
- Clopidogrel: most common
- Prasugrel: more potentn, quicker, increased bleeding risk.
- Ticagrelor: more potent, faster onset, can cause dyspnea but usually self limited
- PLATO trial: ticagrelor with lower mortality rate,
- Clopidogrel in thrombolytics
- clopidogrel, prasugrel, or ticagrelor can be used for PCI
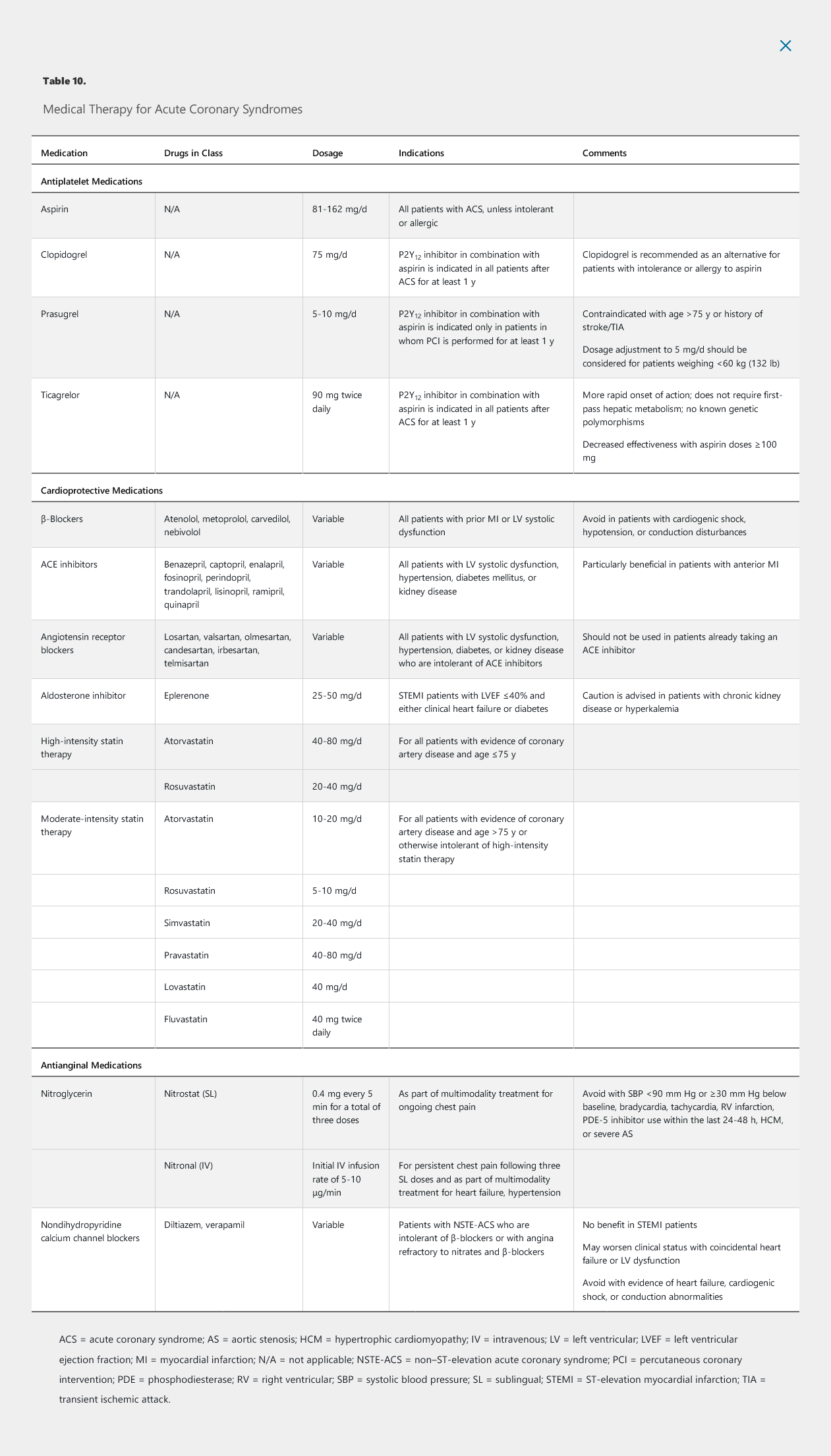
- BB: decrease O2 demand, arrhythmias, improve survival.
- Start in 24 hours
- IV metoprolol reduce early risk of infarct and Vfib but higher rate of cardiogenic shock
- do not give if evidence of hypotension, shock, pulmonary congestion, AV blockade
- ACE I: in most with STEMI and especially with HFrEF, anterior wall infarct, start in 24 hours
- Eplerenone: aldosterone antagonist. Good for STEMI with EF < 40%, good if started within 1 week
- high dose statin
- IV nitro: for HTN. No oral nitro for recovering STEMI
- CCB/ranolazine: do not use in acute STEMI
- do not give O2 for STEMI with normal O2, increases mortality
Complications
Arrhythmias commonly occur in the peri-infarct setting. Atrial fibrillation, which affects up to 10% to 20% of patients with STEMI, complicates management and may cause hemodynamic instability. Ventricular tachycardia and fibrillation may also occur during MI or after reperfusion. Repetitive and sustained bouts of postinfarct ventricular arrhythmias may warrant consultation with an electrophysiologist, as predischarge implantable cardioverter-defibrillator therapy has a role in treating late arrhythmias complicating STEMI. Routine suppression of ventricular ectopy with antiarrhythmic agents is generally not recommended and is associated with increased risk for ventricular arrhythmias. In particular, accelerated idioventricular rhythm, which commonly arises after reperfusion, is generally benign and transient, requiring no treatment. Atrioventricular blocks, including Wenckebach and complete heart block, may occur after inferior MIs. Temporary transvenous pacemakers are sometimes necessary, but permanent pacing is rarely required. Benign forms of vagally mediated heart block must be differentiated from Mobitz type 2 second-degree atrioventricular block, which is more frequently observed with anterior infarction and damage to the conduction system. Mobitz type 2 block may progress to complete heart block and necessitates permanent pacing.
Cardiogenic shock is a common complication of STEMI. It typically results from a large anterior MI due to severely reduced left ventricular systolic function and carries a mortality rate of 50% to 80%. Patients with cardiogenic shock, particularly those younger than 75 years, have a higher rate of survival if they receive emergent revascularization. In these cases, an intra-aortic balloon pump (IABP) or left ventricular assist device may be implanted temporarily, although limited data support their benefits in cardiogenic shock. Once the patient is stabilized, weening the patient from mechanical and inotropic support and gentle uptitration of afterload-reducing agents, such as captopril, can be attempted. β-Blockers should be avoided initially and can be introduced once the patient is stabilized. Diuretics should be used to treat pulmonary vascular congestion.
Approximately 10% to 20% of cases of anterior STEMI are complicated by left ventricular apical thrombus. Although not supported by rigorous studies, anticoagulation with warfarin is generally recommended for at least 3 months to reduce the risk for systemic embolization.
Rates of mechanical complications after STEMI, including left ventricular free wall rupture, right ventricular infarction, ventricular septal defect (VSD), and acute mitral regurgitation, are low; however, clinicians must be able to recognize these complications, given their highly morbid nature. Free wall rupture produces sudden-onset chest pain or syncope with rapid progression to pulseless electrical activity. It is more common in older adults, women, patients with anterior MI, those receiving anti-inflammatory agents, and patients with a significant delay in receiving reperfusion therapy (>12 hours). Surgery should be considered, but mortality rates, even among those who survive to the operating room, are very high.
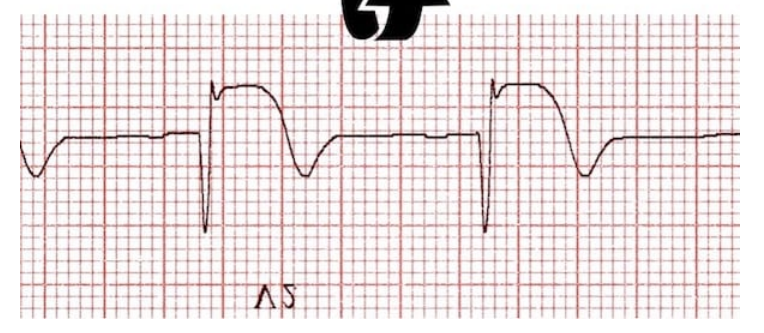
Right ventricular infarction, typically indicated by ST-segment elevation in right-sided ECG leads (V1 and V4R), can complicate right coronary artery occlusion. It presents with hypotension, elevated jugular venous pressure, and an absence of findings on lung auscultation. Right ventricle pump dysfunction causes inadequate filling of the left ventricle, resulting in shock. Treatments include volume resuscitation and positive inotropes (dobutamine or dopamine) to bridge the right ventricle to recovery, which generally takes 2 to 3 days. Nitrates are contraindicated because they may worsen hypotension by reducing preload.
Acquired VSD from septal wall rupture may complicate inferior or anterior STEMI. With inferior STEMI, the VSD tends to be located in the inferior basal septum, whereas anterior STEMI generally leads to an apical VSD. VSDs typically occur within 3 to 5 days of STEMI presentation. Patients present with worsening heart failure and shock, and a harsh holosystolic murmur may be heard at the left lower sternal border. The diagnosis is confirmed with echocardiography. Although initial management may include afterload reduction with medical therapy and IABP support, the mortality rate in patients with medically treated postinfarct VSDs approaches 100%. Surgical closure should be considered; however, the mortality rate in surgical series is still high (approximately 50%). Patch closure can be very difficult owing to the necrotic tissue and inability to find viable myocardium to suture and patch. Percutaneous closure with a VSD occluder device is possible but often unsuccessful because of the nature of the defect, and residual shunting around the device is common.
Acute severe mitral regurgitation may occur as a result of papillary muscle rupture. Most often, the posteromedial papillary muscle ruptures with right coronary artery occlusion. This complication tends to occur several days after STEMI. Afterload reduction and IABP placement may be tried, but urgent surgical intervention is usually necessary. Acute severe mitral regurgitation may also result from left ventricular dysfunction and is often related to an inferior MI with restriction of the posterior mitral leaflet, termed functional ischemic mitral regurgitation. Ischemic mitral regurgitation is treated with revascularization and medical therapy.
Non–ST-Elevation Acute Coronary Syndromes
- pathogenesis: plaque rupture with transient or incomplete occlusion
- Sx: acute chest pain, with rest or minimal exertion
Risk Stratification
- TIMI and GRACE risk models
- TIMI: predicts 14 day death, recurrent MI, urgent revascularization rates
Medical Therapy
Antiplatelet Medications
- ASA: full dose at presentation, followed by daily dose
- Clopidogrel: recommended early loading but unsure timing for loading
- continue one year after NSTE-ACS
- Prasugrel: only in pts with PCI
- Continue DAPT > 1 year for pts with depressed LV, saphenous vein graft stent, DM
- glycoprotein 2b/3a (tirofiban, eptifibatide): used less now. High risk for bleed. Reserved for during PCI
Anticoagulant Medications
- IV heparin or lovenox
- Continue until revascularization
- if no PCI: continue at least 48 hours and generally until discharge
Antianginal Medications
- beta blocker: within 24 hours of presentation
- reduce ventricular arrhythmias, long term mortality
- do not give if evidence of heart failure, shock, bradycardia, heart block, PR > 240
- CCM: for pts with NSTE-ACS intolerant of BB or despite nitrates and BB
- do not give if in shock, has pulmonary edema, or conduction disease
- short acting dihydropyridine contraindicated because can lower BP and increase HR
- nitrates: for sx
- sublingual on presentation
- IV nitro
Lipid-Lowering Medications
- high intensity statin
- load prior to PCI associated with lower periprocedural MI
Invasive Versus Ischemia-Guided Treatment
Immediate invasive treatment (within 2 hours) is recommended for patients with NSTE-ACS who have hemodynamic instability, refractory chest pain, heart failure, or ventricular arrhythmias. In patients with an elevated clinical risk score, significant ST-segment deviation, or elevated cardiac biomarkers, cardiac catheterization is usually performed within 24 hours of presentation. The type of revascularization procedure (PCI or CABG) depends on the results of angiography.
An invasive strategy improves the composite clinical endpoint of death, recurrent MI, and repeat hospitalization compared with an ischemia-guided approach in high-risk and troponin-positive patients with NSTE-ACS. An invasive strategy is the favored approach, with the exception of patients with extensive noncardiac comorbid conditions (such as cancer), in whom the clinical benefits of revascularization may be lower, and patients with acute chest pain unlikely to be related to CAD.
With an ischemia-guided treatment strategy, patients undergo noninvasive stress testing before hospital discharge; cardiac catheterization is reserved for patients with active or intermittent ischemia, including those with angina despite medical therapy or evidence of ischemia on stress testing, and patients at very high clinical risk based on risk score. The ischemia-guided approach is appropriate for low-risk patients (TIMI score <2 or GRACE score <109).
Acute Coronary Syndromes Not Associated with Obstructive Coronary Disease
Elevations in cardiac enzymes, particularly cardiac troponins, coupled with ECG changes provide excellent diagnostic discrimination for ACS. However, some patients with chest pain, elevated cardiac troponin levels, and characteristic ST-segment elevation on ECG have myocardial infarction in the absence of obstructive coronary artery disease (MINOCA). Other related syndromes may mimic MINOCA but do not involve frank infarction. Regardless of whether there is irreversible myocardial injury, treatment of these conditions with thrombolytic agents and revascularization in the absence of occlusive coronary disease and plaque rupture is not beneficial or recommended.
Patients with accelerated hypertension, significant left ventricular hypertrophy, and cardiomyopathies may present with chest pain and elevated cardiac troponin levels caused by elevated left ventricular filling pressures or wall tension rather than plaque rupture and true myocardial infarction. The ECG findings are often abnormal in these patients. Patients with supraventricular tachycardias, which may also dramatically increase the rate-pressure product, often present with chest pain, ST-segment depressions, and elevated cardiac enzyme levels, even if no CAD is present.
Coronary vasospasm is sudden constriction of a coronary artery. It may occur spontaneously or follow use of illicit substances (methamphetamines, cocaine) or prescription drugs (5-fluorouracil, bromocriptine). ECG abnormalities may be nonspecific or mimic STEMI patterns. Coronary vasospasm is a diagnosis of exclusion. Unless the patient has a history of vasospasm, patients often undergo coronary angiography, which may reveal normal findings or slowed coronary flow resulting from microvascular dysfunction. Provocative testing can be performed but is not usually indicated. Patients suspected of having vasospasm (or the related microvascular dysfunction) are usually treated empirically with nitrates and/or calcium channel blockers.
Takotsubo cardiomyopathy, alternatively termed stress cardiomyopathy or apical ballooning syndrome, is a relatively uncommon form of ACS (see Heart Failure). Patients present with acute chest pain, ECG changes (often ST-segment elevations), and elevated cardiac enzyme levels. Takotsubo cardiomyopathy most commonly occurs in women, and there is often, but not always, an antecedent psychological or physical stressor. Patients may initially be diagnosed with STEMI but found to have no significant coronary stenosis at the time of cardiac catheterization. Systolic apical ballooning and notable sparing of the base of the heart on echocardiography or ventriculography are characteristic of this syndrome.
Cardiac syndrome X is a poorly defined condition characterized by anginal chest pain in the presence of angiographically normal coronary arteries or insignificant CAD (<50% stenosis). Cardiac syndrome X is a frequent cause of chest pain syndromes in women, and patients often present without traditional risk factors for CAD. Several hypotheses have been proposed to explain the pathogenesis of this syndrome. One of the most accepted centers on microvascular dysfunction as the cause. Patients may be treated with β-blockers, calcium channel blockers, and nitrates.
Patients with chronic inflammatory muscle diseases or neuromuscular diseases may have elevated levels of cardiac troponin T due to expression of this enzyme in skeletal muscle. The cardiac troponin I level is normal in these cases, which can be helpful in differentiating ACS from these other entities.
Care After an Acute Coronary Syndrome
All patients with ACS should continue aspirin, preferably 81 mg/d, indefinitely. DAPT is recommended for at least 1 year (see Table 10). There is some evidence for extending DAPT beyond 1 year in stented and medically treated patients; however, the decision to prolong therapy should be individualized, with the risk for bleeding weighed against the risk for thrombosis.
Statin therapy should continue indefinitely. Patients with a history of multiple major atherosclerotic cardiovascular disease (ASCVD) events or one major ASCVD event and multiple high-risk conditions are judged to be at very high risk for recurrent ASCVD events. These patients may benefit from the addition of nonstatin drug therapy to maximally tolerated statin therapy. (See MKSAP 18 General Internal Medicine for a discussion on the combined use of statin and nonstatin drugs in selected higher-risk and very-high-risk patients.) Patients younger than 50 years with STEMI have a nearly 10% risk for probable or definite familial hypercholesterolemia, and screening for familial hypercholesterolemia should be considered in this population.
β-Blockade and ACE inhibitor therapy should also be continued indefinitely in patients with left ventricular dysfunction; continuation of these medications is reasonable in patients with normal left ventricular function. Guidelines recommend avoiding NSAIDs if possible, owing to the increased cardiovascular risk associated with these drugs.
Patients should be referred for cardiac rehabilitation, a medically observed exercise program, which reduces mortality while improving functional capacity and risk factor profiles. Patients at low risk (aged ≤75 years with symptoms less than New York Heart Association functional class III to IV, a normal ejection fraction, and no arrhythmia) who prefer home-based cardiac rehabilitation with remote coaching and indirect exercise supervision will benefit as well.
Management of Coronary Artery Disease in Special Populations
Women
Clinical Presentation
Women usually develop ischemic heart disease at an older age than men and more commonly present with stable CAD than an ACS. In women with typical angina symptoms, nonobstructive coronary stenoses are present on coronary angiography in more than 50% of cases, and microvascular dysfunction (endothelium-dependent or endothelium-independent) is thought to be a predominant cause of symptoms in these patients. In women with acute MI, the predominant symptom is chest pain or pressure; however, women can often have atypical symptoms, such as fatigue, dyspnea, nausea, or abdominal symptoms.
Several unique manifestations of cardiovascular disease, including spontaneous coronary artery dissection, takotsubo cardiomyopathy, and coronary vasospasm, occur primarily in women. Spontaneous coronary artery dissection is a common cause of chest pain among younger women who present with ACS. In many cases, spontaneous coronary artery dissection occurs in the peripartum period and is thought to be caused by hormonal changes, although the true cause is unknown. Given the preponderance of young women with this condition, minimizing radiation exposure and avoiding invasive angiography are recommended. This can typically be achieved with the use of supportive care with or without CT angiography. In severe cases, vessel occlusion causes STEMI and necessitates emergent revascularization.
Evaluation and Treatment
Noninvasive stress testing for the evaluation of CAD symptoms has a lower sensitivity and specificity in women than in men, and ST-segment deviation has a lower reported accuracy in women. Therefore, stress testing with imaging provides better accuracy in women (see Diagnostic Testing in Cardiology). Despite these differences, the same guideline recommendations apply to both women and men.
Reports from observational studies and substudies of randomized controlled trials suggest that women have worse outcomes after STEMI presentation. The cause of these worse outcomes is thought to be delays in recognition of CAD and longer overall ischemic time. Complication rates are also reported to be higher in women who undergo reperfusion therapy for STEMI. The COURAGE trial demonstrated that among patients with stable angina treated with revascularization, women had lower rates of overall mortality and nonfatal MI but a higher rate of complications compared with men. Overall, treatment guidelines do not differ for men and women.
Diabetes Mellitus
Risk and Evaluation
Diabetes has been proposed as a CAD equivalent because age-adjusted risk for CAD events is two- to threefold higher in patients with diabetes. Cardiovascular morbidity and mortality are also significantly higher in this population, especially in patients with type 2 diabetes. Much of the risk has been attributed to the higher incidence of known cardiovascular risk factors; however, evidence suggests that underlying vascular dysfunction may play an important role.
Patients with diabetes may present with atypical cardiac symptoms, such as dyspnea or nausea, requiring a high index of suspicion for CAD during their evaluation. The diagnostic accuracy of noninvasive stress testing in symptomatic patients with diabetes is similar to that in patients without diabetes. Although traditional risk factors for CAD should be aggressively managed in patients with diabetes, screening for CAD in asymptomatic persons is controversial, and routine stress testing is not recommended.
Medical Therapy and Secondary Prevention
Medical therapy for patients with diabetes and CAD includes aggressive risk factor reduction, glucose control, and antianginal therapy. The American College of Cardiology/American Heart Association recommend antihypertensive treatment with a target blood pressure below 130/80 mm Hg in patients with diabetes. The American Diabetes Association (ADA) notes that a blood pressure target of less than 130/80 mm Hg may be appropriate for patients with either existing ASCVD or a 10-year ASCVD risk of 15% or greater if it can be safely obtained. For patients at lower ASCVD risk, a blood pressure target of less than 140/90 mm Hg is recommended. ACE inhibitors and ARBs are preferred in the setting of hypertension because of their kidney-protective effects. High-intensity statin therapy is indicated in most patients with diabetes and CAD.
Aspirin is recommended for secondary prevention in all patients with diabetes and CAD.
Tight glycemic control reduces microvascular complications; however, it does not reduce the risk for MI. The use of sodium-glucose cotransporter-2 (SGLT2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists in patients with type 2 diabetes has been shown to reduce rates of acute MI, stroke, and cardiovascular death. For patients with type 2 diabetes and clinical ASCVD, SGLT2 inhibitors may reduce hospitalization for heart failure. These benefits seem to be unrelated to their glucose-lowering effects. Based on strong evidence, the ADA recommends introducing an SGLT2 inhibitor or a GLP-1 receptor agonist with demonstrated cardiovascular disease benefit as part of a glycemic control regimen in patients with type 2 diabetes and clinical ASCVD. If the patient is already taking metformin combined with another therapeutic agent or agents and not taking an SGLT2 inhibitor or GLP-1 receptor agonist, the ADA recommends considering switching to one of these agents.
Initial studies suggested thiazolidinediones, specifically rosiglitazone, were associated with an elevated risk for cardiovascular events, although a subsequent clinical trial demonstrated no elevated risk for MI or death. Consequently, the FDA has removed the restriction on rosiglitazone use in patients with type 2 diabetes and CAD. Metformin does not have any cardiovascular effects, but caution should be exercised in patients undergoing coronary angiography, patients who have had an MI, and patients with heart failure because of concern for potentially fatal lactic acidosis.
Invasive Treatment
The choice of revascularization strategy (PCI or CABG) in patients with diabetes is based on many factors, including the severity and extent of CAD, comorbid conditions, and degree of atherosclerotic narrowing of small, distal vessels. Mortality rates are similar between the two procedures; however, CABG is generally preferred because it is associated with lower rates of repeat revascularization. In patients who undergo PCI, drug-eluting stent placement is recommended to reduce the occurrence of target vessel revascularization because of higher rates of restenosis in patients with diabetes.
PCI timing
Prompt coronary reperfusion with PCI or fibrinolytic therapy restores blood flow, limits myocardial damage, and reduces mortality. Current guidelines recommend primary PCI for patients with acute STEMI as follows:
- Within 12 hours of symptom onset AND
- Within 90 minutes from first medical contact to device time at a PCI-capable facility OR
- Within 120 minutes from first medical contact to device time at a non-PCI-capable facility (to allow time for transport to a PCI-capable facility)
All such patients referred for emergency PCI should be pretreated with antiplatelet and anticoagulant therapy.